商业反式β-硝基苯乙烯类似物的比色氰化物(CN−)检测,通过比较研究,DFT和条带法验证了Michael加成化学剂量法
IF 4.7
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2025-09-13
DOI:10.1016/j.jphotochem.2025.116786
引用次数: 0
摘要
利用商业材料进行有毒氰化物(CN−)阴离子的比色检测,可以大大提高安全性,造福社会。本研究讨论了市售的反式β-硝基苯乙烯类似物,包括反式β-硝基苯乙烯(P1)、反式-4-甲氧基-β-硝基苯乙烯(P2)、反式-4-甲基-β-硝基苯乙烯(P3)、反式-4-氟-β-硝基苯乙烯(P4)、反式-4-溴-β-硝基苯乙烯(P5)、反式-4-氯-β-硝基苯乙烯(P6)和反式-β-甲基-硝基苯乙烯(P7)在二甲基亚砜(DMSO)和乙腈(ACN)中的比色传感性能。P1-P4在检测DMSO和ACN中的CN -离子时呈现出强烈的红粉色和黄橙色,分别在515 nm/510 nm和490 nm/485 nm处出现新的紫外可见峰。相反,P5和P6在DMSO和ACN中对CN -表现出温和的颜色响应,分别在505 nm/510 nm和490 nm/430 nm处出现吸光度峰。由于空间和电子结构的影响,P7对CN -离子没有选择性。P1-P4对CN−的高选择性被干扰研究证实。pH值为6和7是理想的感官测试。P1-P6对CN−的响应在0.1 ~ 1000 μM (μM = 10−6 M)范围内呈线性,估计检测限(lod)为10−9 ~ 10−6 M。核磁共振(NMR)、质谱和密度泛函理论(DFT)分析验证了Michael加成是传感机制。测试条法证明了P1-P3对CN−离子的固态比色传感能力。在水样中加入的CN−离子显示出P1-P4的实时传感能力。这些结果为未来使用硝基(-NO2)迈克尔受体的不同荧光团的设计打开了大门。本文章由计算机程序翻译,如有差异,请以英文原文为准。
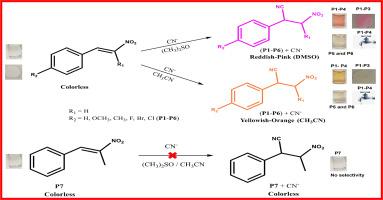
Commercial trans-β-nitrostyrene analogues for colorimetric cyanide (CN−) detection via Michael addition-based chemodosimetric approach validated by comparative investigations, DFT, and strip method
Using commercial materials for colorimetric detection of toxic cyanide (CN−) anions can greatly improve safety and benefit society. This work discusses the colorimetric sensing properties of commercially available trans-β-nitrostyrene analogues, including trans-β-nitrostyrene (P1), trans-4-methoxy-β-nitrostyrene (P2), trans-4-methyl-β-nitrostyrene (P3), trans-4-fluoro-β-nitrostyrene (P4), trans-4-bromo-β-nitrostyrene (P5), trans-4-chloro-β-nitrostyrene (P6), and trans-β-methyl-β-nitrostyrene (P7), in dimethyl sulfoxide (DMSO) and acetonitrile (ACN). P1-P4 show strong reddish-pink and yellowish-orange colors while detecting CN− ions in DMSO and ACN, with new UV–visible peaks appearing at 515 nm/510 nm and at 490 nm/485 nm, respectively. Conversely, P5 and P6 exhibit mild color responses to CN− in DMSO and ACN, with absorbance peaks at 505 nm/510 nm and at 490 nm/430 nm, respectively. P7 shows no selectivity for CN− ions due to steric and electronic structural effects. The high selectivity of P1-P4 for CN− is confirmed through interference studies. pH values of 6 and 7 are ideal for sensory testing. The sensor response of P1-P6 to CN− is linear across a range of 0.1 to 1000 μM (μM = 10−6 M), with estimated detection limits (LODs) at 10−9–10−6 M. Nuclear Magnetic Resonance (NMR), mass spectra, and density functional theory (DFT) analyses validate the Michael addition as the sensing mechanism. The test strip method demonstrates the solid-state colorimetric sensing ability of P1-P3 for CN− ions. Spiked CN− ions in water samples show the real-time sensing capability of P1-P4. These results open the door for future designs using different fluorophores with nitro (-NO2) Michael acceptor.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


