增强光催化甲烷部分氧化制甲醇:2D/金属氧化物复合催化剂的实验研究
IF 4.7
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2025-09-08
DOI:10.1016/j.jphotochem.2025.116765
引用次数: 0
摘要
本研究探讨了光催化过程将甲烷选择性转化为有价值的液体燃料,特别是甲醇的潜力,解决了对高效、替代和清洁能源载体和燃料的需求。合成了两种原始的光催化剂W₁₈O₄₉和g-C₃N₄,以及它们各自的复合剂W₁₈O₄₉/g-C₃N₄,并使用SEM、TEM、XRD、XPS、BET和UV-VIS光谱技术对其进行了分析。此外,通过瞬态光电流测量和电化学阻抗谱(EIS)来阐明光催化剂的电荷转移机理和界面性质。在可见光和紫外光条件下对其光催化性能进行了评价。在测试的三种催化剂中,复合W₁₈O₄₉/g- c₃N₄在可见光下的催化活性为62.3 μmol g−1 h−1,比原始W₁₈O₄₉高近4倍,比g- c₃N₄高5倍。增强的性能归因于Z-scheme异质结体系的形成,XPS分析表明氧空位密度增加,组分之间的电子耦合强,电化学表征进一步证实了电荷分离效率的提高。该复合系统为光催化甲烷转化提供了一种可持续且具有成本效益的贵金属基催化剂替代品,在清洁能源生产和温室气体减排方面显示出巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
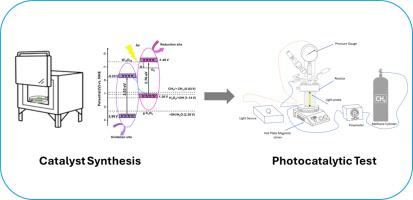
Enhancing photocatalytic partial oxidation of methane to methanol: Experimental investigation of 2D/metal oxide composite catalysts
This study investigates the potential of photocatalytic processes for the selective conversion of methane into valuable liquid fuels, particularly methanol, addressing the need for efficient, alternative, and clean energy carriers and fuels. Two pristine photocatalysts, W₁₈O₄₉ and g-C₃N₄, along with their respective composite W₁₈O₄₉/g-C₃N₄, were synthesized and analyzed using SEM, TEM, XRD, XPS, BET, and UV-VIS spectroscopy techniques. Additionally, transient photocurrent measurements and electrochemical impedance spectroscopy (EIS) were conducted to elucidate charge transfer mechanisms and interfacial properties of the photocatalysts. The photocatalytic performance was evaluated under both visible and UV light conditions. Among the three catalysts tested, the composite W₁₈O₄₉/g-C₃N₄ demonstrated remarkable catalytic activity of 62.3 μmol g−1 h−1 under visible light, nearly 4-fold higher than pristine W₁₈O₄₉ and 5-fold higher than g-C₃N₄. The enhanced performance is attributed to the forming of a Z-scheme heterojunction system, as evidenced by XPS analysis showing increased oxygen vacancy density and strong electronic coupling between the components, further confirmed by electrochemical characterization revealing improved charge separation efficiency. This composite system offers a sustainable and cost-effective alternative to noble metal-based catalysts for photocatalytic methane conversion, demonstrating significant potential for clean energy production and greenhouse gas mitigation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


