高碱度、高杂质溶液溶剂萃取法制备高纯铷盐
IF 9.8
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
铷及其化合物在先进技术中得到广泛应用。碱浸是一种有效的从矿石中提取铷的方法。然而,渗滤液的特点是高碱度和高钾浓度,这使得铷的回收变得复杂。本研究利用t-BAMBP作为萃取剂,实现了对碱性浸出液中铷的高效萃取分离,最终获得高纯度的铷盐产品。首先对稀释剂类型、NaOH浓度、t-BAMBP浓度、混合时间、相比等关键参数进行系统优化。4级逆流萃取验证,Rb提取率为99.3%,萃余液中Rb残留浓度为0.002 g/L。1H NMR和FT-IR分析表明,萃取机理为t-BAMBP中酚羟基上Rb+和H+的阳离子交换。随后,HCl被用作洗涤和剥离剂。经过9级逆流洗涤模拟,负载有机相中的钾浓度降至0.004 g/L,实现了铷和钾的高效分离。最终合成了高纯度的RbCl和Rb2CO3,两者的纯度均达到99.5%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
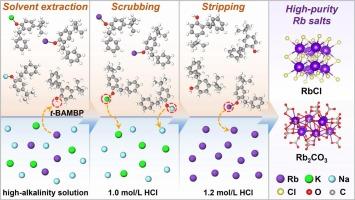
High-purity rubidium salts production from high-alkalinity and high-impurity solution via solvent extraction
Rubidium and its compounds are widely utilized in advanced technologies. Alkaline leaching is an effective approach for rubidium extraction from ores. However, the leachate is characterized by high alkalinity and potassium concentrations, which complicates the recovery of rubidium. This study utilizes t-BAMBP as an extractant to achieve efficient extraction and separation of rubidium from alkaline leachates, ultimately yielding high-purity rubidium salt products. Firstly, key parameters including diluent type, NaOH concentration, t-BAMBP concentration, mixing time, and phase ratio were systematically optimized. A four-stage countercurrent extraction validation achieved 99.3 % Rb extraction, with residual Rb concentration of 0.002 g/L in the raffinate. 1H NMR and FT-IR analyses revealed that the extraction mechanism was cation exchange between Rb+ and H+ on the phenolic hydroxyl group in t-BAMBP. Subsequently, HCl was utilized as both the scrubbing and stripping agent. After a nine-stage countercurrent scrubbing simulation, the potassium concentration in the loaded organic phase decreased to 0.004 g/L, achieving an efficient separation of rubidium and potassium. Ultimately, high-purity RbCl and Rb2CO3 were synthesized, both meeting the purity requirement of 99.5 %.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Desalination
工程技术-工程:化工
CiteScore
14.60
自引率
20.20%
发文量
619
审稿时长
41 days
期刊介绍:
Desalination is a scholarly journal that focuses on the field of desalination materials, processes, and associated technologies. It encompasses a wide range of disciplines and aims to publish exceptional papers in this area.
The journal invites submissions that explicitly revolve around water desalting and its applications to various sources such as seawater, groundwater, and wastewater. It particularly encourages research on diverse desalination methods including thermal, membrane, sorption, and hybrid processes.
By providing a platform for innovative studies, Desalination aims to advance the understanding and development of desalination technologies, promoting sustainable solutions for water scarcity challenges.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


