I科假单胞菌脂肪酶LipGoM的异源表达和折叠酶辅助重折叠
IF 2.2
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
来自假单胞菌的脂肪酶是有价值的生物催化剂,但它们的异源表达往往由于需要不同的方案来确保适当的折叠和活性而变得复杂。在这项研究中,我们成功地生产了一种可溶性和活性形式的LipGoM,一种来自假单胞菌的脂肪酶,在尿素中溶解后采用重折叠策略。我们的研究结果表明,在其天然折叠酶LifGoM存在的情况下,重新折叠LipGoM对于恢复其酶活性至关重要。此外,我们发现,优化再折叠条件,特别是pH值、离子强度、蛋白质浓度、甘油、还原剂和离子的添加,是成功的关键。脂肪酶-脂质GoM混合物Lip-Lif GoM在55°C和pH 8.0条件下对辛酸对硝基苯的水解活性最大,在洗涤剂和有机溶剂中表现出显著的稳定性。这些结果证明了其同源折叠酶LifGoM在激活LipGoM中不可或缺的作用,以及缓冲成分,特别是甘油,对酶的有效折叠及其后续活性的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
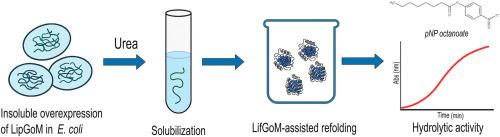
Heterologous expression and foldase-assisted refolding of LipGoM, a Pseudomonas lipase from family I
Lipases from Pseudomonas species are valuable biocatalysts, but their heterologous expression is often complicated by the need for different protocols to ensure proper folding and activity. In this study, we successfully produced a soluble and active form of LipGoM, a lipase derived from Pseudomonas sp., that employs a refolding strategy after solubilization in urea. Our findings indicate that refolding LipGoM in the presence of its native foldase, LifGoM, is essential for restoring its enzymatic activity. Furthermore, we identified that optimizing the refolding conditions, specifically pH, ionic strength, protein concentration, and the addition of glycerol, reducing agents, and ions, was critical for success. The lipase-lipid GoM mixture, Lip-Lif GoM, exhibited maximal hydrolytic activity toward p-nitrophenyl-octanoate at 55 °C and pH 8.0, demonstrating notable stability in the presence of detergents and organic solvents. These results demonstrate the indispensable role of its cognate foldase, LifGoM, in activating LipGoM, as well as the significance of buffer composition, particularly glycerol, for the effective refolding of the enzyme and its subsequent activity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemistry and Biophysics Reports
Biochemistry, Genetics and Molecular Biology-Biophysics
CiteScore
4.60
自引率
0.00%
发文量
191
审稿时长
59 days
期刊介绍:
Open access, online only, peer-reviewed international journal in the Life Sciences, established in 2014 Biochemistry and Biophysics Reports (BB Reports) publishes original research in all aspects of Biochemistry, Biophysics and related areas like Molecular and Cell Biology. BB Reports welcomes solid though more preliminary, descriptive and small scale results if they have the potential to stimulate and/or contribute to future research, leading to new insights or hypothesis. Primary criteria for acceptance is that the work is original, scientifically and technically sound and provides valuable knowledge to life sciences research. We strongly believe all results deserve to be published and documented for the advancement of science. BB Reports specifically appreciates receiving reports on: Negative results, Replication studies, Reanalysis of previous datasets.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


