N6-α-叠氮乙酰辛酮亚胺:叠氮-炔环加成反应的合成及反应活性
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
提出了一种简便的制备方法,用于合成未知的N6-α-叠氮乙酰基辛酮亚胺,并对其在叠氮-炔环加成反应中的反应活性进行了研究。在CuAAC反应的基础上,合成了辛酮亚胺与受保护的葡萄糖、异香豆素和硫辛酸残基的缀合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
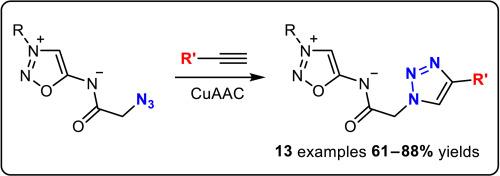
N6-α-azidoacetyl sydnone imines: Synthesis and reactivity in azide-alkyne cycloaddition reactions
A convenient preparative method for the synthesis of previously unknown N6-α-azidoacetyl sydnone imines was developed, and their reactivity in azide – alkyne cycloaddition reactions was investigated. Based on CuAAC reactions, sydnone imine conjugates with protected glucose, isocoumarin, and a lipoic acid residue were synthesized.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


