由稻谷蛋白和大豆蛋白形成的异源淀粉样纤维复合物:组装行为和理化性质
IF 11
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
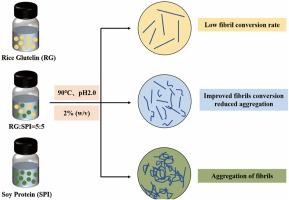
大米谷蛋白(RG)的纤颤效率低,大豆分离蛋白(SPI)的聚集倾向强,限制了它们的实际应用。本研究研究了RG/SPI共纤行为,以阐明所得到的异源淀粉样蛋白原纤维的结构特征和理化性质。结果表明,5:5的RG/SPI混合物的纤维转化率比单独RG高37.31%。此外,增加RG比例减轻了SPI原纤维的聚集,提高了分散均匀性。结构分析表明,所有样品在纤颤后都形成了更有序的β-片结构。值得注意的是,异种淀粉样蛋白原纤维的交叉β片结构比单一淀粉样蛋白原纤维排列更紧密。异种淀粉样原纤维的粘度、储存模量(G′)和损失模量(G″)均低于单淀粉样原纤维,提高了样品的流动性。综上所述,异种淀粉样原纤维的高转化率和低聚集倾向有望在提高生物活性传递效率和改善功能材料性能方面发挥更重要的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Heterologous amyloid fibril complexes formed by rice glutelin and soy protein: Assembly behavior and physicochemical properties
The low fibrillation efficiency of rice glutelin (RG) and the strong aggregation tendency of soy protein isolate (SPI) limit their practical applications. This study investigated RG/SPI co-fibrillation behavior to elucidate the structural features and physicochemical properties of the resulting heterologous amyloid fibrils. Results demonstrated that the 5:5 RG/SPI mixture achieved a 37.31 % higher fibril conversion rate than RG alone. Furthermore, increasing RG proportion mitigated the aggregation of SPI fibrils, enhancing dispersion uniformity. Structural analysis revealed that all samples developed more ordered β-sheet structures after fibrillation. Notably, the cross-β-sheet structure in heterologous amyloid fibrils was more compactly arranged than in single-amyloid fibrils. The viscosity, storage modulus (G′) and loss modulus (G″) of heterologous fibrils were lower than those of single-amyloid fibrils, improving the fluidity of samples. In conclusion, the high conversion rate and low aggregation tendency of heterologous amyloid fibrils are expected to play a more significant role in enhancing the efficiency of bioactive delivery and improving the performance of functional materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Hydrocolloids
工程技术-食品科技
CiteScore
19.90
自引率
14.00%
发文量
871
审稿时长
37 days
期刊介绍:
Food Hydrocolloids publishes original and innovative research focused on the characterization, functional properties, and applications of hydrocolloid materials used in food products. These hydrocolloids, defined as polysaccharides and proteins of commercial importance, are added to control aspects such as texture, stability, rheology, and sensory properties. The research's primary emphasis should be on the hydrocolloids themselves, with thorough descriptions of their source, nature, and physicochemical characteristics. Manuscripts are expected to clearly outline specific aims and objectives, include a fundamental discussion of research findings at the molecular level, and address the significance of the results. Studies on hydrocolloids in complex formulations should concentrate on their overall properties and mechanisms of action, while simple formulation development studies may not be considered for publication.
The main areas of interest are:
-Chemical and physicochemical characterisation
Thermal properties including glass transitions and conformational changes-
Rheological properties including viscosity, viscoelastic properties and gelation behaviour-
The influence on organoleptic properties-
Interfacial properties including stabilisation of dispersions, emulsions and foams-
Film forming properties with application to edible films and active packaging-
Encapsulation and controlled release of active compounds-
The influence on health including their role as dietary fibre-
Manipulation of hydrocolloid structure and functionality through chemical, biochemical and physical processes-
New hydrocolloids and hydrocolloid sources of commercial potential.
The Journal also publishes Review articles that provide an overview of the latest developments in topics of specific interest to researchers in this field of activity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


