α, β-不饱和醛诱导的乳蛋白修饰和多相食品系统中多酚干预的界面效应的新见解
IF 11
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
脂质过氧化是乳制品加工和储存过程中不可避免的挑战。脂质过氧化过程中产生的α, β-不饱和醛与蛋白质发生高活性反应,会降低乳制品的稳定性和营养品质。多酚被广泛用作抗氧化剂来控制α, β-不饱和醛的降解。然而,大多数研究都集中在均相体系上,忽视了乳制品中固有的复杂界面对α、β-不饱和醛修饰蛋白质和多酚干预的影响。在此,我们建立了一个六元-乳蛋白界面模型系统,并使用可点击探针(炔- acr)模拟了α, β-不饱和醛对乳蛋白的修饰。此外,加入6种不同结构的多酚干预炔- acr修饰。结果表明,炔- acr改性和不同多酚的干预均显著改变了乳蛋白的动态界面行为和膨胀流变性能。然而,对界面模型系统稳定性的影响很小。值得注意的是,乳蛋白中的酪蛋白主要吸附在界面上。凝胶内荧光成像显示,界面的存在增强了炔- acr对乳蛋白的修饰作用。多酚的干预作用因其结构特征而异:PA、GA、GeA、RA和EC抑制炔- acr对非吸附β-乳球蛋白(β-Lg)的修饰,而EGCG显著抑制炔- acr对界面蛋白和非吸附蛋白的修饰,并显著降低蛋白羰基化。本研究强调了界面微环境在影响α, β-不饱和醛-蛋白质相互作用中的关键作用,并分析了不同结构的多酚在复杂食物系统中的干预作用。研究结果为乳品加工中合理使用抗氧化策略提供了理论依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
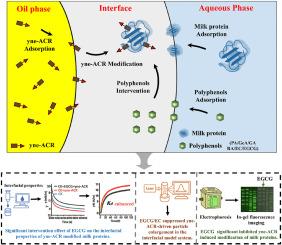
Novel insights into interfacial effects on α, β-unsaturated aldehyde-induced modifications of milk proteins and polyphenol interventions in multiphase food systems
Lipid peroxidation remains an inevitable challenge during dairy processing and storage. The α, β-unsaturated aldehydes produced during lipid peroxidation are highly reactive with proteins, which can degrade the stability and nutritional quality of dairy products. Polyphenols are widely used as antioxidants to control degradation caused by α, β-unsaturated aldehydes. However, most studies focus on homogeneous systems, overlooking the influence of the complex interfaces inherent in dairy products on protein modification by α, β-unsaturated aldehydes and the intervention of polyphenols. Herein, we established a Hex-milk protein interfacial model system and employed clickable probes (yne-ACR) to simulate the modification of milk proteins by α, β-unsaturated aldehydes. Additionally, six polyphenols with different structures were added to intervene the modification by yne-ACR. Results demonstrated that both yne-ACR modification and the intervention of different polyphenols significantly altered the dynamic interfacial behavior and expansion rheological properties of milk proteins. However, the impact on the stability of the interface model system was minimal. Notably, casein in milk proteins predominantly adsorbed at the interface. The in-gel fluorescence imaging revealed that the presence of the interface enhanced the modification of milk proteins by yne-ACR. The intervention effects of polyphenols varied based on their structural characteristics: PA, GA, GeA, RA, and EC inhibited the modification of non-adsorbed β-lactoglobulin (β-Lg) by yne-ACR, while EGCG significantly suppressed yne-ACR modification of both interfacial and non-adsorbed proteins and notably reduced protein carbonylation. This study highlights the critical role of interface microenvironment in influencing α, β-unsaturated aldehyde-protein interactions and analyzes the intervention effects of polyphenols with varying structures in complex food systems. These findings provide a theoretical foundation for the rational use of antioxidant strategies in dairy processing.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Hydrocolloids
工程技术-食品科技
CiteScore
19.90
自引率
14.00%
发文量
871
审稿时长
37 days
期刊介绍:
Food Hydrocolloids publishes original and innovative research focused on the characterization, functional properties, and applications of hydrocolloid materials used in food products. These hydrocolloids, defined as polysaccharides and proteins of commercial importance, are added to control aspects such as texture, stability, rheology, and sensory properties. The research's primary emphasis should be on the hydrocolloids themselves, with thorough descriptions of their source, nature, and physicochemical characteristics. Manuscripts are expected to clearly outline specific aims and objectives, include a fundamental discussion of research findings at the molecular level, and address the significance of the results. Studies on hydrocolloids in complex formulations should concentrate on their overall properties and mechanisms of action, while simple formulation development studies may not be considered for publication.
The main areas of interest are:
-Chemical and physicochemical characterisation
Thermal properties including glass transitions and conformational changes-
Rheological properties including viscosity, viscoelastic properties and gelation behaviour-
The influence on organoleptic properties-
Interfacial properties including stabilisation of dispersions, emulsions and foams-
Film forming properties with application to edible films and active packaging-
Encapsulation and controlled release of active compounds-
The influence on health including their role as dietary fibre-
Manipulation of hydrocolloid structure and functionality through chemical, biochemical and physical processes-
New hydrocolloids and hydrocolloid sources of commercial potential.
The Journal also publishes Review articles that provide an overview of the latest developments in topics of specific interest to researchers in this field of activity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


