黄离子对催化3-芳基环丁酮不对称baeyer-villiger氧化的结构-选择性关系
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
采用黄基离子对催化体系实现了3-芳基环丁酮的立体选择性Baeyer-Villiger氧化反应。催化剂和底物结构的系统变化揭示了关键的结构-选择性关系。与原始催化剂相比,具有单一立体中心的简化催化剂表现出更好的或相当的对映选择性。在芳香环上具有氢键供体取代基的底物表现出增强的对映选择性,最佳的催化剂-底物组合可提供高达99% ee的γ-内酯产物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
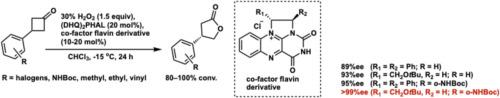
Structure–selectivity correlation in asymmetric baeyer–villiger oxidation of 3-arylcyclobutanones by a flavinium ion-pair catalyst
The stereoselective Baeyer–Villiger oxidation of 3-arylcyclobutanones was achieved using a flavinium-based ion-pair catalyst system. Systematic variation of catalyst and substrate structures revealed key structure–selectivity relationships. A simplified catalyst bearing a single stereocenter demonstrated improved or comparable enantioselectivity relative to the original catalyst. Substrates bearing substituents capable of hydrogen–bond donor substituents on the aromatic ring showed enhanced enantioselectivity, with the optimal catalyst–substrate combination affording the γ-lactone product in up to 99% ee.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


