铜氧化铁促进了2′-硝基查尔酮和NH吡唑之间的级联环
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
首次报道了在氧化铁铜的辅助下,吡唑中2′-硝基查尔酮与NH键级联合成3-氨基吲哚衍生物。我们的方法将提供一种替代方法,以避免强碱和/或不可回收的过渡金属盐的要求。利用XRD、FT-IR和N2物理吸附对废氧化铁铜进行表征,发现在反应过程中材料的结构没有发生变化。还尝试了底物的范围,确认了几种弱亲核吡唑的相容性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
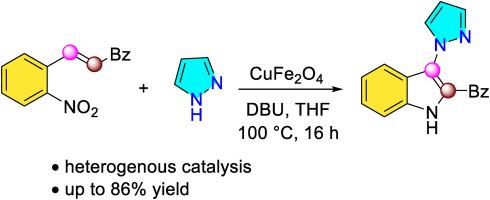
Copper iron oxide promoted cascade annulation between 2′-nitrochalcones and NH pyrazoles
Synthesis of 3-aminoindole derivatives via a cascade coupling of 2′-nitrochalcones and N![]() H bonds in pyrazoles under the assistance of copper iron oxide is firstly reported. Our approach would provide an alternative to avoid the requirement of strong base and/or unrecoverable transition metal salt. Characterization of the spent copper iron oxide by XRD, FT-IR, and N2 physisorption revealed that the structure of the material did not change during the reaction. Scope of the substrates was also attempted, confirming the compatiblity of several weakly-nucleophilic pyrazoles.
H bonds in pyrazoles under the assistance of copper iron oxide is firstly reported. Our approach would provide an alternative to avoid the requirement of strong base and/or unrecoverable transition metal salt. Characterization of the spent copper iron oxide by XRD, FT-IR, and N2 physisorption revealed that the structure of the material did not change during the reaction. Scope of the substrates was also attempted, confirming the compatiblity of several weakly-nucleophilic pyrazoles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


