硫酸葡聚糖增强卵转铁蛋白热稳定性的依赖机制:结构和分子动力学见解
IF 11
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
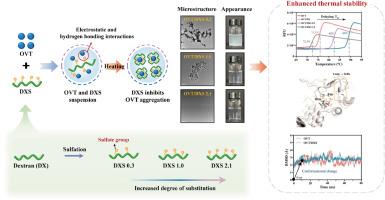
硫酸盐多糖作为一种类似伴侣的物质,可以增强卵转铁蛋白(OVT)的热稳定性,OVT是蛋清中对热最敏感的蛋白质。然而,葡聚糖硫酸盐(DXS)增强OVT热稳定性的分子机制尚不清楚。本文合成了三种磺化度(0.3、1.0和2.1)的DXS,研究磺化程度如何影响其与OVT的相互作用,从而影响结构-稳定性关系。通过分子动力学模拟研究了DXS与OVT的特异性相互作用。浊度、粒径分布和形貌结果表明,DXS作为分子伴侣,有效抑制OVT的热诱导非晶态聚集,并以硫酸依赖的方式促进可溶性低聚物结构的形成。热移实验进一步表明,DXS显著延迟了OVT的熔化温度,在DXS 1.0时提高了22.8%,超过了仪器在DXS 2.1时的检测极限。此外,光谱表征表明,DXS(尤其是DXS 2.1)在热处理过程中较好地保留了OVT的二级和三级结构,且硫酸盐取代度较高,稳定性较好。此外,分子动力学模拟结果在ASN-672、TRP-464和SER-674残基形成的口袋处发现了一个优先结合位点,DXS的结合促进了柔性环区向稳定螺旋结构的转变。DXS与OVT的结合以静电相互作用和氢键为主。这些发现为多糖介导的蛋白质稳定的分子基础提供了新的见解,并支持硫酸多糖作为有效的热保护剂在蛋白质基食品体系中的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Sulfation-dependent mechanism of dextran sulfate enhancing ovotransferrin thermal stability: Structural and molecular dynamics insights
Sulfated polysaccharides have emerged as chaperone-like agents for enhancing the thermal stability of ovotransferrin (OVT), the most heat-sensitive protein in egg white. However, the molecular mechanism by which dextran sulfate (DXS) enhances the thermal stability of OVT remains unclear. Here, we synthesized DXS with three degrees of sulfation (0.3, 1.0, and 2.1) to investigate how the sulfation level influences its interaction with OVT and thereby affects the structure-stability relationship. The specific interaction between DXS and OVT was investigated through molecular dynamics simulations. Turbidity, particle size distribution, and morphology results revealed that DXS acts as a molecular chaperone, effectively inhibiting thermal-induced amorphous aggregation of OVT and promoting the formation of soluble oligomeric structures in a sulfation-dependent manner. Thermal shift assay further showed that DXS significantly delayed the melting temperature of OVT, with a 22.8 % improvement at DXS 1.0 and beyond the instrument's detection limits at DXS 2.1. Besides, spectroscopic characterization revealed that DXS (particularly DXS 2.1) well preserved OVT's secondary and tertiary structures during thermal treatment, with a higher sulfate substitution degree leading to greater stabilization. Furthermore, molecular dynamics simulation results identified a preferential binding site at the pocket formed by ASN-672, TRP-464, and SER-674 residues, where DXS binding promoted the transformation of the flexible loop region into stable helical structures. Electrostatic interactions and hydrogen bonds dominated the binding between DXS and OVT. These findings provide new insights into the molecular basis of polysaccharide-mediated protein stabilization and support the application of sulfated polysaccharides as effective thermal protectants in protein-based food systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Hydrocolloids
工程技术-食品科技
CiteScore
19.90
自引率
14.00%
发文量
871
审稿时长
37 days
期刊介绍:
Food Hydrocolloids publishes original and innovative research focused on the characterization, functional properties, and applications of hydrocolloid materials used in food products. These hydrocolloids, defined as polysaccharides and proteins of commercial importance, are added to control aspects such as texture, stability, rheology, and sensory properties. The research's primary emphasis should be on the hydrocolloids themselves, with thorough descriptions of their source, nature, and physicochemical characteristics. Manuscripts are expected to clearly outline specific aims and objectives, include a fundamental discussion of research findings at the molecular level, and address the significance of the results. Studies on hydrocolloids in complex formulations should concentrate on their overall properties and mechanisms of action, while simple formulation development studies may not be considered for publication.
The main areas of interest are:
-Chemical and physicochemical characterisation
Thermal properties including glass transitions and conformational changes-
Rheological properties including viscosity, viscoelastic properties and gelation behaviour-
The influence on organoleptic properties-
Interfacial properties including stabilisation of dispersions, emulsions and foams-
Film forming properties with application to edible films and active packaging-
Encapsulation and controlled release of active compounds-
The influence on health including their role as dietary fibre-
Manipulation of hydrocolloid structure and functionality through chemical, biochemical and physical processes-
New hydrocolloids and hydrocolloid sources of commercial potential.
The Journal also publishes Review articles that provide an overview of the latest developments in topics of specific interest to researchers in this field of activity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


