负载金催化剂催化吲哚的c3选择性CH硼化反应
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
负载型Au催化剂有效地促进了吲哚中C(sp2) -H键的硼化反应。该反应在C3位置上具有很高的区域选择性,可以得到收率很高的C3硼化吲哚。值得注意的是,负载的Au催化剂在多个循环中表现出可重复使用性,并且反应很容易扩展到克级而不会损失效率。机制研究表明,硼化是通过金纳米粒子的单电子转移催化产生的自由基中间体的形成进行的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
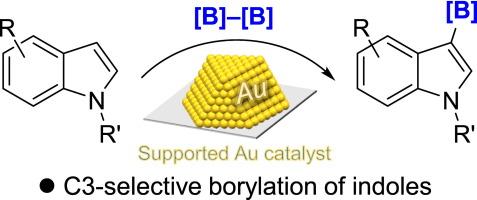
C3-selective CH borylation of indoles by supported gold catalysts
Supported Au catalysts efficiently promoted the borylation of C(sp2)–H bonds in indoles. The reaction proceeded with high regioselectivity at the C3 position, affording C3-borylated indoles in good to excellent yields. Notably, the supported Au catalysts demonstrated reusability over multiple cycles, and the reaction was readily scalable to the gram scale without loss of efficiency. Mechanistic studies suggest that the borylation proceeds via the formation of radical intermediates, generated by single electron transfer catalysis of Au nanoparticles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


