光诱导芳胺的区域选择性硫氰化和硒氰化
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在这里,我们报道了一个区域选择性氧化C(sp2) -H硫氰化/硒氰化芳胺使用市售和廉价的NaSCN/KSeCN作为硫/硒源。该方法采用非过渡铋催化剂在可见光照射下,通过光致配体到金属电荷转移(LMCT)均解机制工作。通过芳胺氮与铋配位形成的瞬态BiN配合物在辐照下发生lmct触发的均溶性BiN键裂解。这产生了一个氮中心自由基中间体,通过区域选择性分子内氢原子转移(HAT)异构化为对芳基自由基。随后与硫氰酸酯/硒氰酸酯亲核试剂的自由基交叉偶联形成具有完全对选择性的CS或CSe键。该方案的特点是温和的条件,广泛的官能团耐受性,并避免化学计量氧化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
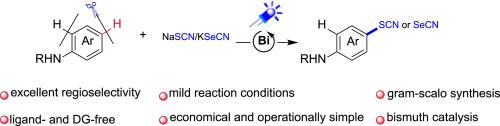
Photoinduced regioselective CH thiocyanation and selenocyanation of arylamines
Herein we report a regioselective oxidative C(sp2)–H thiocyanation/selenocyanation of arylamines using commercially available and inexpensive NaSCN/KSeCN as sulfur/selenium sources. This method employs a non-transition bismuth catalyst under visible-light irradiation, operating through a photoinduced ligand-to-metal charge transfer (LMCT) homolysis mechanism. The transient Bi![]() N complex, formed via coordination of the arylamine nitrogen to bismuth, undergoes LMCT-triggered homolytic Bi
N complex, formed via coordination of the arylamine nitrogen to bismuth, undergoes LMCT-triggered homolytic Bi![]() N bond cleavage upon irradiation. This generates a nitrogen-centered radical intermediate that isomerizes to a para-aryl radical via regioselective intramolecular hydrogen atom transfer (HAT). Subsequent radical cross-coupling with thiocyanate/selenocyanate nucleophiles forges C
N bond cleavage upon irradiation. This generates a nitrogen-centered radical intermediate that isomerizes to a para-aryl radical via regioselective intramolecular hydrogen atom transfer (HAT). Subsequent radical cross-coupling with thiocyanate/selenocyanate nucleophiles forges C![]() S or C
S or C![]() Se bonds with exclusive para-selectivity. The protocol features mild conditions, broad functional group tolerance, and avoids stoichiometric oxidants.
Se bonds with exclusive para-selectivity. The protocol features mild conditions, broad functional group tolerance, and avoids stoichiometric oxidants.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


