二硫代氨基甲酸酯-羟酸盐捕收剂在nacl -氧化浮选体系中提高方解石与红锰矿的分离效率
IF 5
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
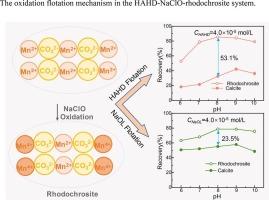
由于红锰矿和方解石的溶解特性,使得它们的表面性质相似,这使得从方解石中分离红锰矿更加困难。因此,采用表面改性的方法将红锰矿石上的Mn(II)物质氧化到更高的价态,降低了Mn2+的溶解度,使其表面性质与方解石不同。次氯酸钠(NaClO)是提高菱锰矿可浮性的合适氧化剂。同时,N-[(3-羟基氨基)-丙氧基]-N-己基二硫代氨基甲酸酯(HAHD)是一种多功能捕收剂,可氧化生成Gemini分子(HAHD)2,在氯化钠氧化的红锰矿-方解石人工混合矿物浮选体系中具有选择性。HAHD在混合矿物氧化浮选中的选择性指数IMn达到32.2,远高于油酸钠的选择性指数(9.9)。有趣的是,紫外光谱和XPS分析表明,在氧化浮选体系中,HAHD被氧化成Gemini结构(HAHD)2。在HAHD- nacl -红锰矿浮选体系中,发生氧化还原反应,即红锰矿表面Mn(II)首先被氧化为Mn(IV), Mn(IV)将HAHD氧化为(HAHD)2,然后部分Mn(IV)种被还原为Mn(II)。最后提出了一种潜在的机理,揭示了方解石混合物中红锰矿选择性浮选的本质。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Intensifying the separation efficiency of rhodochrosite from calcite in NaClO-oxidation flotation system with a dithiocarbamate-hydroxamate collector
The dissolution characteristics of rhodochrosite and calcite results in their similar surface properties, which makes the separation of rhodochrosite from calcite more difficult. Therefore, surface modification was adopted to oxidize the Mn(II) species on rhodochrosite to higher valence states, reducing the solubility of Mn2+ and differentiating the surface properties from calcite. Sodium hypochlorite (NaClO) was a suitable oxidant to enhance the floatability of rhodochrosite. Meanwhile, N-[(3-hydroxyamino)-propoxy]-N-hexyl dithiocarbamate (HAHD) was a versatile collector, which can be oxidized into a Gemini molecule of (HAHD)2 and featured a selective performance in the NaClO-oxidized flotation system of rhodochrosite-calcite artificially mixed minerals. The selectivity index IMn of HAHD in the oxidized flotation of mixed minerals reached 32.2, much higher than that of sodium oleate (9.9). Interestingly, UV spectra and XPS analyses indicated that HAHD was oxidized to a Gemini structure (HAHD)2 in the oxidized flotation system. In HAHD-NaClO-rhodochrosite flotation system, REDOX reaction occurred, that was, the surface Mn(II) of rhodochrosite was first oxidized to Mn(IV) which oxidized HAHD to (HAHD)2, then some of Mn(IV) species were reduced to Mn(II). A potential mechanism was proposed finally to reveal the essence of the selective flotation of rhodochrosite from the mixture with calcite.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Minerals Engineering
工程技术-工程:化工
CiteScore
8.70
自引率
18.80%
发文量
519
审稿时长
81 days
期刊介绍:
The purpose of the journal is to provide for the rapid publication of topical papers featuring the latest developments in the allied fields of mineral processing and extractive metallurgy. Its wide ranging coverage of research and practical (operating) topics includes physical separation methods, such as comminution, flotation concentration and dewatering, chemical methods such as bio-, hydro-, and electro-metallurgy, analytical techniques, process control, simulation and instrumentation, and mineralogical aspects of processing. Environmental issues, particularly those pertaining to sustainable development, will also be strongly covered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


