废催化剂中钒的超高效水热酸浸工艺
IF 5
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
从废选择性催化还原(SCR)催化剂中回收钒是一个关键的挑战。本研究探索了水热酸浸法提取钒的新方法,分析了浸出剂浓度、液固比、反应温度、浸出时间等关键参数。采用Box-Behnken设计(BBD)和响应面法,研究了这些参数对钒浸出效率的影响及其相互作用。机制上,硫酸浓度决定了钒溶解成VO2+/VO2+,而过量的酸或不适当的液固比会导致钛析出(TiOSO4/Ti(SO4)2),从而包裹颗粒并捕获钒离子。结果表明,浸出剂浓度优化通过抑制这些副反应显著提高了钒的回收率。液固比与浸出时间、液固比与反应温度的交互作用对浸出效果有显著影响。最佳浸出条件为2.13 mol·L−1 H2SO4, 23.85 mL·g−1,143℃,89 min,钒浸出率为99.76%。XRD和SEM-EDS显示浸渣结构稳定,表明其具有后续金属回收的潜力。该研究强调了水热酸浸法是一种可持续的钒回收方法,有助于节约资源和减少废物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
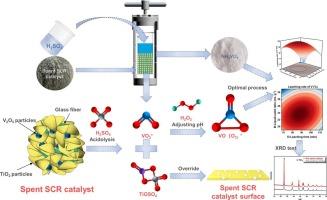
Hydrothermal acid leaching process with ultra-high efficiency for vanadium from spent catalyst
Recovery of vanadium from spent selective catalytic reduction (SCR) catalysts is a critical challenge. This study explores hydrothermal acid leaching as a novel approach for extracting vanadium, analyzing key parameters such as leaching agent concentration, liquid–solid ratio, reaction temperature, and leaching time. Using the Box-Behnken design (BBD) and response surface method, the effects and interactions of these parameters on vanadium leaching efficiency were investigated. Mechanistically, sulfuric acid concentration governs vanadium dissolution into VO2+/VO2+, while excessive acid or improper liquid–solid ratios induce titanium precipitation (TiOSO4/Ti(SO4)2) that encapsulates particles and captures vanadium ions. The results showed that leaching agent concentration optimization significantly enhanced vanadium recovery by suppressing these side reactions. The interaction between liquid–solid ratio and leaching time, as well as liquid–solid ratio and reaction temperature, significantly affected efficacy. Optimal conditions were determined as 2.13 mol · L−1 H2SO4, 23.85 mL · g−1, 143 °C, and 89 min, achieving 99.76 % vanadium leaching. XRD and SEM-EDS revealed stable leaching slag structure, indicating its potential for subsequent metal recovery. This study highlights hydrothermal acid leaching as a sustainable method for vanadium recovery, contributing to resource conservation and waste reduction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Minerals Engineering
工程技术-工程:化工
CiteScore
8.70
自引率
18.80%
发文量
519
审稿时长
81 days
期刊介绍:
The purpose of the journal is to provide for the rapid publication of topical papers featuring the latest developments in the allied fields of mineral processing and extractive metallurgy. Its wide ranging coverage of research and practical (operating) topics includes physical separation methods, such as comminution, flotation concentration and dewatering, chemical methods such as bio-, hydro-, and electro-metallurgy, analytical techniques, process control, simulation and instrumentation, and mineralogical aspects of processing. Environmental issues, particularly those pertaining to sustainable development, will also be strongly covered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


