以BiVO4阳极为主导(040)面,从聚对苯二甲酸乙酯废弃物中制备乙醇酸盐和氢气
IF 9.5
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在这项研究中,研究了在光电化学配置下乙二醇(EGOR)的氧化制氢反应,以减少水分解中的能量势垒。为此,合成了具有暴露(040)面的BiVO4光阳极,随着(040)面组成的增加,EGOR的起始电位降低到0.215 V(相对于RHE),在含n2饱和的碱性电解质中(相对于RHE),在0.5 V时光电流密度为3.55 mA cm - 2。在n2饱和PET塑料碱性溶液中,在0.5 V ~−0.3 V(相对于RHE)的电位窗口内循环伏安,析氢速率达到1.41 μmol cm−2 h−1,比平坦的BVO提高了7.4倍。值得注意的是,在这个电位窗口中,氧化产物被鉴定为乙二醛和乙氧酸盐,产率分别为61.6和134.7 μmol cm−2 h−1。通过DFT计算,BVO板的表面在热力学上有利于EGOR,并且这种配置允许高活性表面积和快速电荷转移动力学。因此,这项工作为通过可持续的方法利用丰富的金属氧化物光电极对PET废物进行升级回收提供了有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
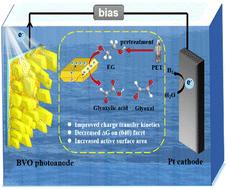
Photoelectrochemical glyoxylates and hydrogen production from polyethylene terephthalate wastes using BiVO4 anode with dominant (040) facets
In this study, the oxidation reaction of ethylene glycol (EGOR) for hydrogen production is investigated in a photoelectrochemical configuration to reduce the energy barrier in water splitting. For this, BiVO4 photoanode with exposed (040) facets is synthesized and with increase of the constitution of (040) facets, the onset potential for EGOR is reduced to 0.215 V (vs. RHE), the photocurrent density is 3.55 mA cm−2 at 0.5 V in N2-saturated EG-containing alkaline electrolyte (vs. RHE). Cyclic voltammetry is then performed in the potential window from 0.5 V to −0.3 V (vs. RHE) in N2-saturated PET plastic alkaline solution, where hydrogen evolution rate reaches 1.41 μmol cm−2 h−1, making a 7.4-fold increase compared to the flat BVO. Notably, in this potential window, the oxidation products are identified to be glyoxal and glyoxylate, with production rates of 61.6 and 134.7 μmol cm−2 h−1, respectively. (040) Facet of BVO plates is thermodynamically favourable for EGOR by DFT calculations, and such configuration allows for high active surface area and fast charge transfer kinetics. This work thus offers valuable insights on upcycling of PET wastes utilizing abundant metal oxide photoelectrodes via a sustainable approach.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Materials Chemistry A
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
19.50
自引率
5.00%
发文量
1892
审稿时长
1.5 months
期刊介绍:
The Journal of Materials Chemistry A, B & C covers a wide range of high-quality studies in the field of materials chemistry, with each section focusing on specific applications of the materials studied. Journal of Materials Chemistry A emphasizes applications in energy and sustainability, including topics such as artificial photosynthesis, batteries, and fuel cells. Journal of Materials Chemistry B focuses on applications in biology and medicine, while Journal of Materials Chemistry C covers applications in optical, magnetic, and electronic devices. Example topic areas within the scope of Journal of Materials Chemistry A include catalysis, green/sustainable materials, sensors, and water treatment, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


