新型细胞周期蛋白依赖性激酶4/6(CDK4/6)和组蛋白去乙酰化酶(HDAC)双抑制剂的设计与合成:体外和体内抗癌活性
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
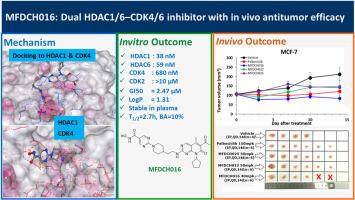
某些肿瘤对CDK4/6抑制剂的耐药性源于细胞周期蛋白D1的上调和细胞周期调节因子p21的下调。相反,HDAC抑制剂可以通过上调乙酰组蛋白H3和p21的表达水平来抵消这种抗性。为了解决这一挑战,我们开发了一系列新的双靶向抑制剂,同时抑制CDK4/6和HDAC1。其中,选定的化合物MFDCH016、MFDCH022和MFDCH025在纳摩尔水平上对CDK4和HDAC1/6有抑制作用,诱导MCF-7细胞G2/M和G0/G1期细胞凋亡和细胞周期阻滞,促进细胞凋亡。这种作用是通过HDAC-p21-CDK信号通路的调节介导的,乙酰- h3和p21水平的升高证明了这一点。化合物MFDCH016对乳腺癌细胞系和MCF-7异种移植瘤模型均有明显的抗肿瘤活性,且无明显毒性。更重要的是,与标准药物帕博西尼相比,MFDCH016对CDK4的选择性更高。我们的数据表明,化合物MFDCH016作为单药可能是一种新的双靶向CDK4/6-HDAC抑制剂,可能是一种有前景的癌症治疗候选药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Design and synthesis of novel cyclin-dependent kinase 4/6(CDK4/6) and histone deacetylase (HDAC) dual inhibitors: In vitro and in vivo anticancer activity
Resistance to CDK4/6 inhibitors in certain tumors arises from the upregulation of cyclin D1 and the downregulation of the cell cycle regulator p21. Conversely, HDAC inhibitors can counteract this resistance by upregulating acetyl-histone H3 and p21 expression levels. To address this challenge, we developed a series of novel dual-targeting inhibitors that simultaneously inhibit CDK4/6 and HDAC1. Among these, selected compounds MFDCH016, MFDCH022 and MFDCH025 potently inhibited CDK4 and HDAC1/6 at nM levels, inducing apoptosis and cell cycle arrest in G2/M and G0/G1 phase and promote apoptosis in MCF-7 cells. This effect was mediated through the modulation of the HDAC-p21-CDK signalling pathway, as evidenced by increased acetyl-H3 and p21 levels. Compound MFDCH016 demonstrated significant anti-tumor activity in breast cancer cell line and in MCF-7 xenograft model without apparent toxicity. More importantly MFDCH016 show highly selective against CDK4 over CDK2 compare to standard drug Palbociclib. Our data demonstrated that compound MFDCH016 as a single agent could be novel dual-targeting CDK4/6-HDAC inhibitor could be a promising drug candidate for cancer therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


