用于4.7 V高压实用锂离子和锂金属电池的双盐添加剂增强商用碳酸盐基电解质
IF 20.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
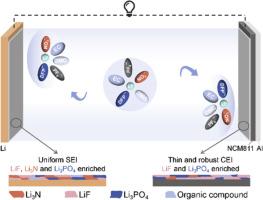
商用六氟磷酸锂(LiPF6)/碳酸盐电解质体系,尽管在锂离子电池技术中占据主导地位超过30年,但仍然受到两个关键缺陷的限制,包括热力学稳定性不足,导致锂枝晶生长过快,高压下阴极-电解质反应严重,再加上LiPF6对水分的不稳定性。本文将0.1 mol L−1 (M)二氟磷酸锂(LiDFP)和0.1 M硝酸锂(LiNO3)的双盐添加剂引入到商用碳酸电解质中。NO3−和DFP−阴离子主要在Li+的第一溶剂化鞘层中占主导地位,同时在电极表面的内Helmholtz平面(IHP)富集,形成以LiF、Li3PO4和Li3N为主的均匀且富无机的界面相,有效地稳定了电极界面。因此,优化后的电解液能够稳定地在石墨阳极中插入/脱嵌Li+,并且可以在4.7 v级Li||NCM811电池中循环500次以上。值得注意的是,由于NO3−和H2O分子之间的强相互作用,这种电解质对水也表现出惊人的稳定性。这种双盐添加剂策略弥合了实验室创新与工业实用性之间的差距,为与现有制造协议兼容的下一代高压锂金属电池提供了可扩展的、具有成本效益的电解质工程解决方案。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Dual-salt-additive reinforced commercial carbonate-based electrolytes for 4.7 V high-voltage practical Li-ion and Li-metal batteries
The commercial lithium hexafluorophosphate (LiPF6)/carbonate electrolyte system, despite dominating lithium-ion battery technologies for over three decades, remains fundamentally constrained by two critical flaws, including insufficient thermodynamic stability, resulting in aggressive lithium dendrites growth and severe cathode-electrolyte reactions at high voltages, coupled with the instability of the LiPF6 against moisture. Herein, we introduce a dual-salt additive of 0.1 mol L−1 (M) lithium difluorophosphate (LiDFP) and 0.1 M lithium nitrate (LiNO3) into the commercial carbonate electrolyte. Both NO3− and DFP− anions mainly dominate in the first solvation sheath of Li+ and simultaneously enrich in the inner Helmholtz plane (IHP) at the electrode surface, leading to the formation of a uniform and inorganic-rich interphase dominated by LiF, Li3PO4, and Li3N, effectively stabilizing electrode interfaces. Consequently, the optimized electrolyte enables stable Li+ intercalation/deintercalation in graphite anodes, and cycling of over 500 cycles for 4.7 V-class Li||NCM811 batteries. Remarkably, due to the strong interaction between NO3− and H2O molecules, this electrolyte also exhibits astonishing stability toward water. This dual-salt additive strategy bridges the gap between laboratory innovation and industrial practicality, offering a scalable, cost-effective electrolyte engineering solution for next-generation high-voltage lithium metal batteries compatible with existing manufacturing protocols.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


