Daurisoline通过直接结合PPARα并阻断其泛素化介导的降解来抑制胰腺癌的进展。
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
背景:胰腺癌(PC)以其高致死率而闻名,目前可用的治疗方案有限。Daurisoline (DAU)是一种双苄基异喹啉生物碱,从dauricummenispermum dauricum DC中分离得到。尽管其确切的作用机制尚不清楚,但已证明对几种癌症有抗肿瘤作用。目的:本研究评估dau介导的胰腺癌抑制作用及其机制。方法:通过EdU、CCK-8、创面愈合、Transwell和流式细胞术检测DAU对PC细胞的抑制作用。生物信息学分析显示,加DAU或不加DAU处理的PC细胞的分子表达发生了变化。通过PPARα慢病毒转导和STAT3激活剂处理,分别研究了PPARα和JAK2/STAT3通路在DAU介导的PC抑制中的作用。采用共免疫沉淀法确认DAU对PPARα泛素化的影响,并鉴定了所涉及的特异性泛素连接酶。结果:我们的研究结果显示,DAU以时间和剂量依赖的方式抑制胰腺癌细胞的增殖,同时抑制细胞的迁移和侵袭。此外,DAU在G1期阻滞细胞周期并诱导细胞凋亡。在机制上,生物信息学分析结合实验验证表明,DAU与PPARα结合,增加其表达,随后抑制JAK2/STAT3信号传导。值得注意的是,DAU通过与PPARα结合,竞争性地阻断Trim63-PPARα相互作用,从而抑制PPARα泛素化,增加其蛋白稳定性。结论:DAU通过抑制PPARα泛素化抑制JAK2/STAT3信号通路,从而抑制PC细胞的进展。本研究为通过抑制泛素化途径治疗PC提供了一种新的治疗策略和有前景的化合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
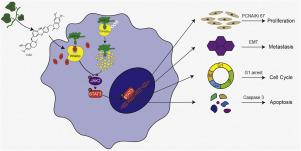
Daurisoline inhibits pancreatic cancer progression by direct binding to PPARα and blocking its ubiquitination-mediated degradation
Background
Pancreatic cancer (PC) is well known for its high lethality, and limited therapeutic options are currently available. Daurisoline (DAU), a bisbenzylisoquinoline alkaloid isolated from Menispermum dauricum DC., has demonstrated antitumor effects in several cancers, although its precise mechanism of action remains unclear.
Purpose
This study evaluated the DAU-mediated inhibition of pancreatic cancer and the mechanisms responsible.
Methods
DAU's inhibitory effects on PC cells were measured through EdU, CCK-8, wound healing, Transwell, and flow cytometry assays. Bioinformatics analysis revealed molecular expression alterations between PC cells treated with or without DAU. The roles of the PPARα and JAK2/STAT3 pathway in DAU-mediated PC suppression were investigated via PPARα lentiviral transduction and STAT3 activator treatment, respectively. Coimmunoprecipitation assays were performed to confirm the impact of DAU on PPARα ubiquitination and identify the specific ubiquitin ligase involved.
Results
Our findings revealed that DAU suppresses the proliferation of pancreatic cancer cells in a time- and dose-dependent manner while also inhibiting cell migration and invasion. Additionally, DAU arrests the cell cycle at the G1 phase and induces apoptosis. Mechanistically, bioinformatics analysis combined with experimental validation demonstrated that DAU binds to PPARα, increases its expression, and subsequently suppresses JAK2/STAT3 signaling. Notably, DAU competitively blocks the Trim63–PPARα interaction by binding to PPARα, thereby suppressing PPARα ubiquitination to increase its protein stability.
Conclusion
DAU inhibits the JAK2/STAT3 signaling pathway by preventing PPARα ubiquitination, thereby suppressing the progression of PC cells. This research provides a new therapeutic strategy and a promising compound for treatment of PC through suppression of the ubiquitination pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


