白曲酸通过ampk介导的Nrf2和FoxO3a抗氧化途径激活,减轻慢性间歇性缺氧诱导的脑损伤。
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
背景:阻塞性睡眠呼吸暂停综合征(OSAS)是一种慢性疾病,影响全球约10%的成年人,在男性和肥胖人群中患病率较高,可导致不可逆的神经认知障碍。白屈酸(AA)是一种从地衣中提取的天然深苷类化合物,具有清除自由基和金属螯合的双重功能。然而,其治疗osas相关病理的潜力仍未被探索。目的:探讨AA对cih诱导的神经损伤的保护作用及其分子机制。方法:建立CIH小鼠模型和HT22神经元缺氧损伤模型。AA分别以5、10、20 mg/kg的剂量(根据文献和初步实验选择)和20 μM的最佳浓度(通过CCK-8测定)给药给小鼠。通过Morris水迷宫、高架迷宫和开阔场地测试等行为测试评估治疗效果。通过组织病理学检查(如海马CA3和DG亚区神经元存活)和氧化应激/铁下垂标志物检测(如MDA水平、GPX4表达和相关生物标志物)评估神经保护作用。转录组测序和分子对接分析用于研究差异表达基因,途径富集及其潜在机制。结果:体内和体外实验表明,AA可显著减轻CIH小鼠神经元损伤,提高HT22细胞活力。AA下调氧化应激和铁中毒相关基因的表达。转录组测序确定AMPK信号是关键靶点。联合AMPK抑制剂(Compound C)和核细胞质分离实验证实,AA通过AMPK活化协同调节双抗氧化途径(Nrf2/HO-1和FoxO3a/SOD2)。结论:AA通过ampk依赖性激活Nrf2/HO-1和FoxO3a/SOD2轴来减轻cih诱导的神经认知障碍,建立双抗氧化-铁下沉防御屏障。该研究首次利用转录组学数据与计算模拟相结合,系统地阐明了AA的分子机制,为开发基于天然化合物的OSAS疗法提供了新的治疗靶点和转化范例。本文章由计算机程序翻译,如有差异,请以英文原文为准。
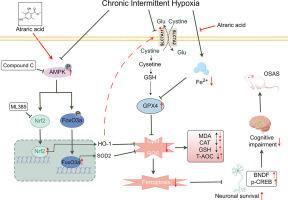
Atraric acid attenuates chronic intermittent hypoxia-induced brain injury via AMPK-mediated Nrf2 and FoxO3a antioxidant pathway activation
Background
Obstructive sleep apnea syndrome (OSAS), a chronic disorder affecting approximately 10 % of adults worldwide with heightened prevalence in males and obese populations, induces irreversible neurocognitive impairment. Atraric Acid (AA), a natural depside compound derived from lichens, exhibits dual functional properties through its molecular groups capable of free radical scavenging and metal chelation. However, its therapeutic potential in OSAS-related pathologies remains unexplored.
Objective
This study aims to investigate the neuroprotective effects and molecular mechanisms of AA against CIH-induced neuronal damage.
Methods
CIH mouse models and hypoxic injury models in HT22 neurons were established. AA was administered to mice at doses of 5, 10, and 20 mg/kg (selected based on literature references and preliminary experiments), and to cells at an optimal concentration of 20 μM (determined by CCK-8 assays). Therapeutic efficacy was evaluated through behavioral tests including the Morris Water Maze, Elevated Plus Maze, and Open Field Test. Neuroprotective effects were assessed via histopathological examination (e.g., neuronal survival in hippocampal CA3 and DG sub-regions) and detection of oxidative stress/ferroptosis markers (e.g., MDA levels, GPX4 expression, and related biomarkers). Transcriptomic sequencing and molecular docking analyses were employed to investigate differentially expressed genes, pathway enrichment, and underlying mechanisms.
Results
In vivo and in vitro experiments demonstrated that AA significantly alleviated neuronal damage in CIH mice and enhanced HT22 cell viability. AA down-regulated oxidative stress- and ferroptosis-related gene expression. Transcriptomic sequencing identified AMPK signaling as a key target. Combined AMPK inhibitor (Compound C) and nuclear-cytoplasmic fractionation experiments confirmed that AA synergistically regulates dual antioxidant pathways (Nrf2/HO-1 and FoxO3a/SOD2) via AMPK activation.
Conclusion
AA mitigates CIH-induced neurocognitive impairment by AMPK-dependent activation of the Nrf2/HO-1 and FoxO3a/SOD2 axes, establishing a dual antioxidant-ferroptosis defense barrier. This study is the first to systematically elucidate AA's molecular mechanisms using transcriptomics data integrated with computational simulations, providing novel therapeutic targets and a translational paradigm for developing natural compound-based therapies for OSAS.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


