二氧化硅纳米颗粒通过减少细胞连接和增加细胞凋亡来抑制体外3D培养小鼠腔前卵泡的发育。
IF 2.8
4区 医学
Q2 REPRODUCTIVE BIOLOGY
引用次数: 0
摘要
二氧化硅纳米颗粒具有良好的化学和物理性能,在农业、生物医药等领域具有广阔的应用前景。人们对snp的生物安全性非常关注。snp可诱导卵泡闭锁,导致卵巢毒性。然而,仅研究卵母细胞或卵巢颗粒细胞似乎不足以充分揭示snp对卵泡发育的影响。建立卵泡体外三维培养系统,研究snp对小鼠卵巢的毒性。通过观察卵泡形态、测定卵泡直径、成熟卵泡数和卵母细胞成熟度来评价卵泡发育。采用酶联免疫吸附法测定17-β雌二醇(E2)分泌量。用phalloidin-iFluor检测微丝分布,用Mitotracker Red检测线粒体分布。结果表明,snp破坏卵巢颗粒细胞排列,抑制其生长,阻碍卵泡腔形成和卵母细胞成熟。单核苷酸多态性显著增加卵巢颗粒细胞凋亡,升高Caspase-3 mRNA水平。此外,snp显著降低E2分泌和STAR、CYP11A1和CYP19A1 mRNA水平。此外,snp导致线粒体分布不均匀,在卵母细胞内形成簇状甚至弥漫性。SNPs也缩短了卵泡中微绒毛的长度和数量。同时,卵母细胞中CX37蛋白和mRNA水平均显著降低。综上所述,snp诱导卵泡闭锁,至少部分是通过抑制卵母细胞成熟和破坏卵母细胞与卵巢颗粒细胞之间的间隙功能。本研究利用体外3D培养的腔前卵泡证实了snp对卵泡的毒性,为snp诱导的卵巢毒性研究提供了新的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
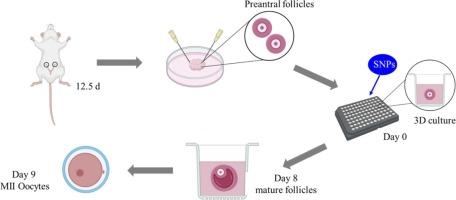
Silica nanoparticles inhibit the development of mouse preantral follicle in vitro 3D culture via a decrease in cell junctions and an increase in cell apoptosis
Silica nanoparticles (SNPs) are promising nanomaterial with desirable chemical and physical properties for their applications in agriculture, biomedicine and others. There are serious concerns regarding the biosafety of SNPs. SNPs can induce follicular atresia, leading to ovarian toxicity. However, studying only oocytes or ovarian granulosa cells seems insufficient to fully reveal the impact of SNPs on the development of follicles. An in vitro 3D culture system of the follicles was established to investigate mouse ovarian toxicity of SNPs. Follicular development was evaluated through observation of follicular morphology, measurement of follicular diameter, number of mature follicles and oocyte maturation. The secretion of 17-β estradiol (E2) was measured by ELISA. Microfilament distribution was examined by phalloidin-iFluor, and mitochondria distribution was detected by Mitotracker Red. The results showed that SNPs disrupted ovarian granulosa cell arrangement, inhibited their growth, and hindered follicular antrum formation and oocyte maturation. SNPs significantly increased cell apoptosis in ovarian granulosa cells and elevated Caspase-3 mRNA level. Additionally, SNPs significantly reduced E2 secretion and STAR, CYP11A1, and CYP19A1 mRNA levels. Furthermore, SNPs caused mitochondria to distribute unevenly, forming clusters or even diffusely within the oocytes. SNPs also shortened the length and reduced the number of microvilli in the follicles. Meanwhile, both CX37 protein and mRNA level were significantly decreased in the oocytes. In summary, SNPs induced follicular atresia, at least partly, via inhibition of oocyte maturation and damage of the gap functions between the oocytes and ovarian granulosa cells. This study confirmed the toxicity of SNPs on follicles using in vitro 3D culture of preantral follicles, providing a new perspective for the study of SNP-induced ovarian toxicity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Reproductive toxicology
生物-毒理学
CiteScore
6.50
自引率
3.00%
发文量
131
审稿时长
45 days
期刊介绍:
Drawing from a large number of disciplines, Reproductive Toxicology publishes timely, original research on the influence of chemical and physical agents on reproduction. Written by and for obstetricians, pediatricians, embryologists, teratologists, geneticists, toxicologists, andrologists, and others interested in detecting potential reproductive hazards, the journal is a forum for communication among researchers and practitioners. Articles focus on the application of in vitro, animal and clinical research to the practice of clinical medicine.
All aspects of reproduction are within the scope of Reproductive Toxicology, including the formation and maturation of male and female gametes, sexual function, the events surrounding the fusion of gametes and the development of the fertilized ovum, nourishment and transport of the conceptus within the genital tract, implantation, embryogenesis, intrauterine growth, placentation and placental function, parturition, lactation and neonatal survival. Adverse reproductive effects in males will be considered as significant as adverse effects occurring in females. To provide a balanced presentation of approaches, equal emphasis will be given to clinical and animal or in vitro work. Typical end points that will be studied by contributors include infertility, sexual dysfunction, spontaneous abortion, malformations, abnormal histogenesis, stillbirth, intrauterine growth retardation, prematurity, behavioral abnormalities, and perinatal mortality.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


