麝香保心丸通过抑制STAT3磷酸化抑制成纤维细胞向肌成纤维细胞转化,改善心肌纤维化。
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
背景:麝香保心丸是一种治疗缺血性心肌病的中药。它可以减轻心肌缺血再灌注(MI/R)损伤后的心肌纤维化,但其作用机制尚不清楚。目的:探讨收缩压减轻心肌纤维化的作用机制。方法:采用snRNA-seq技术构建舒张压处理心肌梗死/R小鼠心肌细胞图谱,鉴定成纤维细胞中的deg,分析转录因子活性。通过分子对接和分子动力学模拟研究筛选的SBP化学成分与STAT3蛋白的结合位点和结合稳定性。采用免疫组织化学、免疫荧光、qRT-PCR和WB技术评估收缩压治疗对小鼠心肌纤维化和肌成纤维细胞转化的影响,超声心动图评价小鼠心功能。结果:舒张压治疗可显著改善心肌梗死/R损伤小鼠的心功能:心肌纤维化面积减少4.2%,EF增加4.5%,FS增加2.5%。snRNA-seq显示收缩压治疗没有改变心脏成纤维细胞的总数,但调节了其亚型的比例分布。基于scenic的转录因子活性分析表明,STAT3信号通路可能在这一过程中发挥关键作用。体外细胞实验表明,H/R处理诱导成纤维细胞向α- sma阳性肌成纤维细胞转变,舒张压抑制了这种转变。结论:本研究首次证明收缩压可有效减轻mi /R后心肌纤维化,并阐明了收缩压通过STAT3信号通路抑制肌成纤维细胞转分化的机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
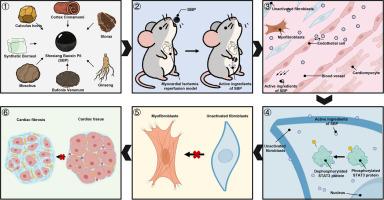
Shexiang baoxin pill ameliorates cardiac fibrosis by inhibiting fibroblast to myofibroblast transition through STAT3 phosphorylation suppression
Background
Shexiang Baoxin Pill (SBP) is a traditional Chinese medicine used to treat ischemic cardiomyopathy. It can alleviate cardiac fibrosis following myocardial ischemia/reperfusion (MI/R) injury, but its action mechanism remains unclear.
Purpose
To elucidate the mechanism by which SBP alleviates cardiac fibrosis.
Methods
snRNA-seq was employed to construct a cardiac cell atlas of MI/R mice treated with SBP, identify DEGs in fibroblasts, and analyse transcription factor activity. Molecular docking and molecular dynamics simulations were used to investigate the binding sites and binding stability of the screened SBP chemical components with the STAT3 protein. Immunohistochemistry, immunofluorescence, qRT-PCR and WB techniques were utilized to assess the impact of SBP treatment on cardiac fibrosis and myofibroblast transition, while echocardiography was used to evaluate cardiac function in mice.
Results
SBP treatment significantly improved cardiac function in mice subjected to MI/R injury: cardiac fibrosis area was reduced by 4.2 %, EF was increased by 4.5 % and FS was increased by 2.5 %. snRNA-seq revealed that SBP treatment did not alter the overall number of cardiac fibroblasts but modulated the proportional distribution of their subtypes. SCENIC-based transcription factor activity analysis suggested that the STAT3 signalling pathway might play a key role in this process. In vitro cell experiments demonstrated that H/R treatment induced the transition of fibroblasts into α-SMA-positive myofibroblasts, which SBP inhibited.
Conclusions
This study has demonstrated for the first time that SBP effectively attenuates cardiac fibrosis post-MI/R, and elucidated the mechanism by which SBP inhibits myofibroblast transdifferentiation via the STAT3 signaling pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


