原发性纤毛完整性在骨关节炎发病过程中调控trpv4介导的NF-κB/COL2信号。
IF 3.7
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
摘要
软骨细胞机械转导功能障碍是骨关节炎(OA)的一个关键特征。已有研究表明,初级纤毛在软骨细胞机械感觉和信号转导中起着至关重要的作用。瞬时受体电位香草蛋白4(Transient Receptor Potential Vanilloid 4, TRPV4)是一种重要的机械转导蛋白,是主要的Ca2+通道之一,在初级纤毛上高度表达。然而,初级纤毛介导的信号传导机制与TRPV4通道调节机制之间的相互作用仍然知之甚少。在这里,我们证明OA软骨细胞和鞭毛内转运蛋白88(Ift88)条件敲除(cKO)小鼠的纤毛发病率降低。这导致软骨变性。TRPV4的表达与纤毛完整性呈负相关,随着纤毛丢失而增加。在纤毛完整的健康软骨细胞中,TRPV4拮抗剂抑制Ca2+依赖性NF-κB的激活,同时促进II型胶原(COL2)的合成。这些作用被白细胞介素-1β (IL-1β)诱导的纤毛缺陷或IFT88缺乏所消除。染色质免疫沉淀(ChIP)分析显示,由于纤毛完整性的丧失,p65与COL2启动子的结合被破坏。至关重要的是,Ift88 cKO软骨细胞对TRPV4通道没有反应,Ca2+通量和NF-κB和COL2表达水平的变化证实了这一点。我们的研究结果表明,初级纤毛是trpv4介导的NF-κ b - COL2信号传导不可或缺的调节因子,为OA发病机制提供了新的见解,并确定了IFT88作为治疗检查点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
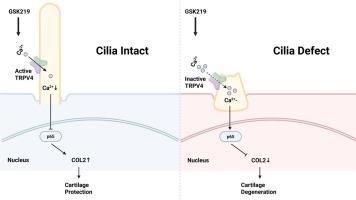
Primary cilia integrity governs TRPV4-mediated NF-κB/COL2 signaling in osteoarthritis pathogenesis
Chondrocyte mechanotransduction dysfunction is a key feature of osteoarthritis (OA). Previous studies have demonstrated that primary cilia play a crucial role in chondrocyte mechanosensation and signal transduction. Transient Receptor Potential Vanilloid 4 (TRPV4), which is highly expressed on primary cilia, is a critical mechanotransduction protein and one of the most important Ca2+ channels. However, the interplay between the mechanism of primary cilia-mediated signaling and that of TRPV4 channel regulation remains poorly understood. Here, we demonstrate that OA chondrocytes and Intraflagellar Transport Protein 88 (Ift88) conditional knockout (cKO) mice exhibit reduced ciliary incidence resulting in cartilage degeneration. TRPV4 expression inversely correlated with ciliary integrity, increasing as cilia were lost. In cilia-intact healthy chondrocytes, TRPV4 antagonism inhibited Ca2+-dependent NF-κB activation while promoting type II collagen (COL2) synthesis. These effects were abolished by Interleukin-1 beta (IL-1β)-induced ciliary defect or IFT88 deficiency. Chromatin immunoprecipitation (ChIP) analysis revealed disrupted p65 binding to the COL2 promoter upon the loss of ciliary integrity. Crucially, Ift88 cKO chondrocytes showed unresponsiveness of the TRPV4 channel, as confirmed by Ca2+ flux and changes in the expression levels of NF-κB and COL2. Our findings position primary cilia as indispensable regulators of TRPV4-mediated NF-κB - COL2 signaling, offering mechanistic insights into OA pathogenesis and identifying IFT88 as a therapeutic checkpoint.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cellular signalling
生物-细胞生物学
CiteScore
8.40
自引率
0.00%
发文量
250
审稿时长
27 days
期刊介绍:
Cellular Signalling publishes original research describing fundamental and clinical findings on the mechanisms, actions and structural components of cellular signalling systems in vitro and in vivo.
Cellular Signalling aims at full length research papers defining signalling systems ranging from microorganisms to cells, tissues and higher organisms.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


