人工智能和放射组学生物标志物用于预测晚期her2阴性乳腺癌的治疗反应。
IF 7.9
2区 医学
Q1 OBSTETRICS & GYNECOLOGY
引用次数: 0
摘要
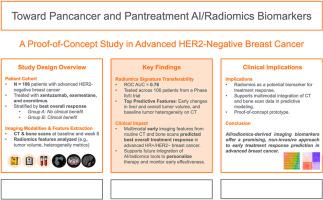
研究目的:尽管有了新的治疗方法,乳腺癌的死亡率仍然很高。需要改进治疗反应评估工具。我们的目的是测试外部验证的AI/放射组学生物标志物的可转移性,以预测晚期、激素受体阳性(HR+)、her2型乳腺癌使用xentuzumab、依西美坦和依维莫司治疗的治疗反应。方法:回顾性分析一项Ib/II期临床试验(2014年5月- 2016年10月)的患者数据。使用AUC分析验证了8种成像生物标志物(肝脏和整体肿瘤体积,代表基线和第8周肿瘤异质性的两个放射组学特征)用于预测临床获益的有效性。一项辅助人工智能分析开发了一个签名,用于预测同一队列中使用40个变量(3个临床变量和37个影像学变量)的最佳总体反应。结果:在106例可获得分析数据的患者中,28例无临床获益(A组),78例有临床获益(B组)。8个成像生物标志物中有7个显示出显著的预测价值。B组患者的肝脏和整体肿瘤体积的基线和随访测量值显著降低,同时肿瘤异质性在第8周发生了显著变化。在我们的辅助AI/放射组学模型中,预测的主要驱动因素是治疗期间肝脏肿瘤和总体肿瘤体积的变化以及基线骨扫描时成骨细胞病变的数量。结论:这项跨癌症概念验证试验研究支持了应用多模态AI/放射组学生物标志物预测晚期HR+、HER2-乳腺癌治疗反应的可行性,为更广泛的癌症和泛治疗应用奠定了基础,有待进一步验证。临床试验注册号:NCT02123823。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Artificial intelligence and radiomics biomarkers for treatment response prediction in advanced HER2-negative breast cancer
Study Aim
Despite the development of novel therapies, breast cancer mortality remains high. Improved treatment response assessment tools are needed. We aimed to test the transferability of externally validated AI/radiomics biomarkers for predicting treatment response in advanced, hormone receptor-positive (HR+), HER2-breast cancer treated with xentuzumab, exemestane, and everolimus.
Methods
Patient data from a phase Ib/II trial (May 2014–October 2016) were analyzed retrospectively. Eight imaging biomarkers (liver and overall tumor volume, two radiomics features representing tumor heterogeneity at both baseline and week 8) were validated for predicting clinical benefit using AUC analysis. An ancillary AI analysis developed a signature for predicting best overall response using 40 variables (3 clinical and 37 imaging) in the same cohort.
Results
Of 106 patients with data available for analysis, 28 had no clinical benefit from treatment (Group A) vs. 78 with clinical benefit (Group B). Seven of eight imaging biomarkers demonstrated significant predictive value. Participants in Group B exhibited significantly lower baseline and follow-up measures of liver and overall tumor volume, alongside marked changes in tumor heterogeneity by week 8. In our ancillary AI/radiomics model, the dominant drivers of prediction were changes in both liver tumor and overall tumor volume during treatment and the number of osteoblastic lesions on baseline bone scans.
Conclusion
This cross-cancer proof-of-concept testing study supports the feasibility of applying multimodal AI/radiomics biomarkers to predict treatment response in advanced HR+, HER2− breast cancer, laying the foundation for broader pancancer and pantreatment applications pending further validation.
Clinical trial registration number
NCT02123823
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Breast
医学-妇产科学
CiteScore
8.70
自引率
2.60%
发文量
165
审稿时长
59 days
期刊介绍:
The Breast is an international, multidisciplinary journal for researchers and clinicians, which focuses on translational and clinical research for the advancement of breast cancer prevention, diagnosis and treatment of all stages.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


