高强度间歇训练通过缓解同型半胱氨酸诱导的Cldn5转录抑制改善慢性应激小鼠的认知功能障碍
IF 3.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
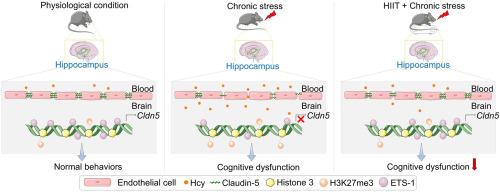
慢性应激性血脑屏障(BBB)功能障碍有助于神经系统疾病,同型半胱氨酸(HCY)是一个关键的危险因素。考虑到药物治疗的局限性,像高强度间歇训练(HIIT)这样的非侵入性干预是有希望的。为了确定HIIT是否能改善应激诱导的血脑屏障功能障碍和认知障碍,我们首先建立了慢性不可预测轻度应激(CUMS)模型,并将小鼠分为四组:Control (Ctrl)、CUMS、HIIT和HIIT + CUMS。在此,我们发现HIIT显著改善了雄性CUMS小鼠的认知功能障碍,这可以通过减少morris水迷宫的逃避潜伏期和提高新物体识别测试的记忆表现来证明。HIIT还通过改善应激小鼠的紧密连接破坏和血脑屏障的超通透性来保持血脑屏障的完整性。随后,为了明确HCY在HIIT介导效应中的作用,我们建立了HHCY模型,并将小鼠分为Ctrl、HIIT、HHCY和HIIT + HHCY四组。结果表明,HIIT通过恢复CBS、MTHFR和MS等相关代谢酶的表达,使血浆和海马HCY水平正常化,减轻了hhcy引起的认知能力下降和血脑屏障损伤。此外,HIIT通过抑制Cldn5 (Cldn5的编码基因)启动子区域的H3K27me3富集,逆转了hcy诱导的Claudin-5下调。此外,HIIT恢复了Cldn5的转录激活因子之一ETS1的表达,促进了Cldn5基因的转录和BBB的稳定。总的来说,这些研究结果表明,HIIT通过消除HHCY对血脑屏障完整性的破坏性影响来改善慢性压力诱导的认知障碍,为压力相关的认知缺陷提供了一种非药物干预的可能性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
High-intensity interval training improves cognitive dysfunction in chronically stressed mice through alleviating homocysteine-induced transcriptional repression of Cldn5
Chronic stress-induced blood-brain barrier (BBB) dysfunction contributes to neurological disorders, with homocysteine (HCY) as a key risk factor. Considering pharmacotherapy limitations, non-invasive interventions like high-intensity interval training (HIIT) are promising. To determine whether HIIT improves stress-induced BBB dysfunction and cognitive impairment, we first established a chronic unpredictable mild stress (CUMS) model and assigned mice into four groups: Control (Ctrl), CUMS, HIIT, and HIIT + CUMS. Here, we found that HIIT significantly ameliorated cognitive impairment in male CUMS mice, as evidenced by reduced escape latency in morris water maze and increased memory performance in novel object recognition test. HIIT also preserved BBB integrity by ameliorating the tight junction disruption and BBB hyper-permeability in stressed mice. Subsequently, to clarify the role of HCY in the HIIT-mediated effects, we established an HHCY model and divided mice into four groups: Ctrl, HIIT, HHCY, and HIIT + HHCY. The results showed that HIIT normalized the plasma and hippocampal HCY levels by restoring the expression of related metabolic enzymes including CBS, MTHFR and MS, and alleviated HHCY-induced cognitive decline and BBB damage. Further, HIIT reversed HCY-induced Claudin-5 downregulation by inhibiting H3K27me3 enrichment at the Cldn5 (the encoding gene of Claudin-5) promoter region. In addition, HIIT restored the expression of ETS1, one of the transcriptional activators of Cldn5, to facilitate the transcription of Cldn5 gene and the stabilization of BBB. Collectively, these findings reveal that HIIT improves chronic stress-induced cognitive impairment via eliminating the disruptive effects of HHCY on the BBB integrity, offering a non-pharmacological intervention potential for stress-related cognitive deficits.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Neurobiology of Stress
Biochemistry, Genetics and Molecular Biology-Biochemistry
CiteScore
9.40
自引率
4.00%
发文量
74
审稿时长
48 days
期刊介绍:
Neurobiology of Stress is a multidisciplinary journal for the publication of original research and review articles on basic, translational and clinical research into stress and related disorders. It will focus on the impact of stress on the brain from cellular to behavioral functions and stress-related neuropsychiatric disorders (such as depression, trauma and anxiety). The translation of basic research findings into real-world applications will be a key aim of the journal.
Basic, translational and clinical research on the following topics as they relate to stress will be covered:
Molecular substrates and cell signaling,
Genetics and epigenetics,
Stress circuitry,
Structural and physiological plasticity,
Developmental Aspects,
Laboratory models of stress,
Neuroinflammation and pathology,
Memory and Cognition,
Motivational Processes,
Fear and Anxiety,
Stress-related neuropsychiatric disorders (including depression, PTSD, substance abuse),
Neuropsychopharmacology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


