石墨等离子体表面工程及其对提高铝电池性能的影响
IF 11.6
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
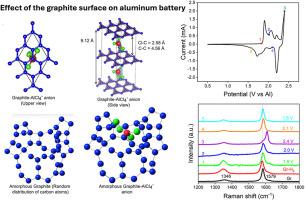
铝电池具有高重量容量和高成本效益的铝金属阳极,是现有储能装置的一个有前途的替代品。石墨由于其高导电性和容纳非水铝电池氯铝酸盐阴离子的能力,在各种探索的阴极活性材料中处于领先地位。然而,石墨的质量、表面化学、污染和各种结构对性能的影响是不同的。特别是石墨表面对铝阴离子的快速嵌入有显著的影响。在这里,我们提出了一种快速简便的等离子体表面工程策略来定制商业石墨薄片,以研究它们对氯铝酸盐阴离子储存能力的影响。采用温和的氢气和氩气等离子体对石墨表面进行了改造,并调整了结构质量。值得注意的是,氢等离子体处理石墨的电化学性能显著提高,在50 mA/g时具有132.68 mAh/g的比容量,在1000 mA/g时具有83.94 mAh/g的高倍率性能和良好的稳定性。非原位拉曼和x射线光电子能谱研究表明,等离子体表面裁剪允许氯铝酸盐的快速插层。石墨的受控等离子体表面处理指导了氯铝酸盐通过石墨表面插入化学的基本原理的基本理解。通过密度泛函理论计算证实了表面处理对离子嵌入和储能能力的影响。这一发现将为利用商用石墨开发实用铝电池铺平新的道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Plasma surface engineering of graphite and its effect on advancing the performance of aluminium battery
Aluminium batteries, with high gravimetric capacities and cost-effective aluminium metal anodes, are a promising alternative to the existing energy storage devices. Graphite is a frontrunner among variously explored cathode active materials due to its high electrical conductivity and ability to accommodate chloroaluminate anions for non-aqueous aluminium batteries. However, the quality of graphite, surface chemistry, contamination, and various structures affect the performance differently. Particularly, the graphite surface significantly influences the fast intercalation of aluminium anions. Here, we put forward a fast and facile plasma-enabled surface engineering strategy to tailor the commercial graphite flakes to investigate their effect on the storage capabilities of chloroaluminate anions. A mild hydrogen and argon plasma was used to engineer the graphite surface and tailor the structural quality. Notably, the hydrogen plasma-treated graphite exhibits a significant increase in electrochemical performance by delivering a remarkable specific capacity (132.68 mAh/g at 50 mA/g) and excellent high-rate performance (83.94 mAh/g at 1000 mA/g) with good stability. Ex-situ Raman and X-ray photoelectron spectroscopy studies showed that plasma surface tailoring allows the fast intercalation of the chloroaluminate. The controlled plasma surface treatment on graphite directs the fundamental understanding of the basic principles of intercalation chemistry of chloroaluminate in graphite via the surface. The effect of the surface treatment on the ion intercalation and energy storage capability was confirmed and demonstrated by the density functional theory calculation. Such a finding would pave a new path to developing practical aluminium batteries using commercially available graphite.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Carbon
工程技术-材料科学:综合
CiteScore
20.80
自引率
7.30%
发文量
0
审稿时长
23 days
期刊介绍:
The journal Carbon is an international multidisciplinary forum for communicating scientific advances in the field of carbon materials. It reports new findings related to the formation, structure, properties, behaviors, and technological applications of carbons. Carbons are a broad class of ordered or disordered solid phases composed primarily of elemental carbon, including but not limited to carbon black, carbon fibers and filaments, carbon nanotubes, diamond and diamond-like carbon, fullerenes, glassy carbon, graphite, graphene, graphene-oxide, porous carbons, pyrolytic carbon, and other sp2 and non-sp2 hybridized carbon systems. Carbon is the companion title to the open access journal Carbon Trends. Relevant application areas for carbon materials include biology and medicine, catalysis, electronic, optoelectronic, spintronic, high-frequency, and photonic devices, energy storage and conversion systems, environmental applications and water treatment, smart materials and systems, and structural and thermal applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


