n掺杂碳量子点诱导的氰富集增强了k掺杂C3N4光催化生成H2O2的效率
IF 11.6
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
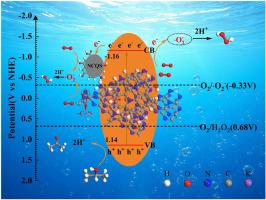
光催化生产过氧化氢(H2O2)是一种清洁、可持续的合成方法。本研究开发了一种协同双策略改性,以实现级联效应,提高大块氮化碳的光催化活性。主要的改进涉及创新地加入氮掺杂碳量子点(NCQS)以促进钾掺杂。NCQS具有双重功能。一方面,它们作为氰基诱导剂,促进掺钾氮化碳结构内部开环形成氰基,从而促进氰基官能团的富集。另一方面,NCQS作为表面改性剂,与K+协同作用,直接增强载流子在层间和表面之间的迁移。二次改性采用碱性水热法引入氮空位(NVs)。结合实验结果和DFT计算可知,富集的氰基和NVs修饰了氮化碳的电子结构,促进了载流子的分离和输运,同时为氧还原反应提供了丰富的氰基活性反应位点。经过修饰后,KCNC5-K的H2O2产率达到12366 μmol g−1 h−1,比g- c3n4提高了107.2倍,H2O2选择性达到80%。该研究不仅提出了一种通过碳量子点的掺入来调节氮化碳表面氰基的新方法,而且为开发用于过氧化氢生产的高性能氮化碳基光催化剂提供了宝贵的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
N-doped carbon quantum dot-induced cyano-enrichment enhances K-doped C3N4 for efficient photocatalytic H2O2 production
Photocatalytic hydrogen peroxide (H2O2) production represents a clean and sustainable synthesis method. In this work, a synergistic dual-strategy modification was developed to achieve cascade effects for enhancing the photocatalytic activity of bulk carbon nitride. The primary modification involved innovative incorporation of nitrogen-doped carbon quantum dots (NCQS) to facilitate potassium doping. The NCQS serve a dual function. On the one hand, they act as cyano-group inducers, promoting ring-opening within the potassium-doped carbon nitride structure to form cyano groups and thereby facilitate cyano functionality enrichment. On the other hand, the NCQS act as a surface modifier that synergized with K+ to directly enhance charge carrier migration across interlayers and surfaces. The secondary modification employed alkaline hydrothermal treatment to introduce nitrogen vacancies (NVs). Combining the experimental results and DFT calculation, it can be seen that the enriched cyano groups and NVs modify the electronic structure of carbon nitride, which promotes the separation and transport of charge carriers while providing abundant cyano-active reaction sites for the oxygen reduction reaction. Benefiting from the modification, The KCNC5–K achieves an outstanding H2O2 production rate of 12,366 μmol g−1 h−1 (a 107.2-fold enhancement over g-C3N4) with a remarkable H2O2 selectivity of 80 %. This study not only proposes a novel approach for modulating the cyano groups on the surface of carbon nitride via incorporation of carbon quantum dots, but also offers valuable insights for the development of high-performance carbon nitride-based photocatalysts for hydrogen peroxide production.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Carbon
工程技术-材料科学:综合
CiteScore
20.80
自引率
7.30%
发文量
0
审稿时长
23 days
期刊介绍:
The journal Carbon is an international multidisciplinary forum for communicating scientific advances in the field of carbon materials. It reports new findings related to the formation, structure, properties, behaviors, and technological applications of carbons. Carbons are a broad class of ordered or disordered solid phases composed primarily of elemental carbon, including but not limited to carbon black, carbon fibers and filaments, carbon nanotubes, diamond and diamond-like carbon, fullerenes, glassy carbon, graphite, graphene, graphene-oxide, porous carbons, pyrolytic carbon, and other sp2 and non-sp2 hybridized carbon systems. Carbon is the companion title to the open access journal Carbon Trends. Relevant application areas for carbon materials include biology and medicine, catalysis, electronic, optoelectronic, spintronic, high-frequency, and photonic devices, energy storage and conversion systems, environmental applications and water treatment, smart materials and systems, and structural and thermal applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


