金刚石中位错成核的电子观点
IF 11.6
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
晶体的塑性变形是由位错的成核和运动控制的,广义层错能(GSFE)为金属的能态成核提供了有效的判据。然而,在像金刚石这样的超硬共价晶体中,高临界弹性应变导致塑性起始点阵构型明显偏离平衡,使得用于GSFE计算的点阵模型不确定,从而限制了传统GSFE准则的适用性。通过分析位错滑动过程中原子构型的演变及其相关电子分布,我们发现位错核心的局部金属化,表现为可转移传导电子的出现,是金刚石中位错滑动的先决条件。我们在金刚石晶格中发现了一个约为1.12 eV的临界带隙,在此带隙以下,具有本禀弹性应变场的位错核心区可能发生局部金属化。该带隙阈值在晶格不稳定之前达到,可以作为任意应力状态下共价材料脆性向延性转变的可推广指标,克服了传统基于gsfe标准的局限性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
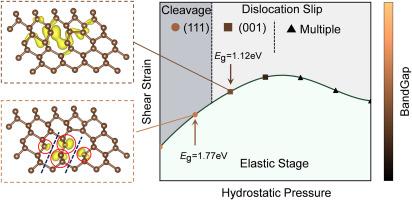
An electronic view on dislocation nucleation in diamond
Plastic deformation in crystals is governed by the nucleation and motion of dislocations, for which generalized stacking fault energy (GSFE) provides an effective energetic nucleation criterion in metals. However, in superhard covalent crystals such as diamond, the high critical elastic strain causes the lattice configuration at plastic initiation to deviate significantly from equilibrium, rendering the lattice model used in GSFE calculations uncertain and thereby limiting the applicability of traditional GSFE criteria. Here, by analyzing the evolution of atomic configurations and their associated electronic distributions during dislocation glide, we show that local metallization at the dislocation core, manifested by the emergence of transferrable conduction electrons, is a prerequisite for dislocation glide in diamond. We identify a critical bandgap of approximately 1.12 eV in the diamond lattice, below which a dislocation core region with intrinsic elastic strain field may undergo local metallization. This bandgap threshold, reached prior to lattice instability, serves as a generalizable indicator of the brittle-to-ductile transition in covalent materials under arbitrary stress states, overcoming the limitations of traditional GSFE-based criteria.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Carbon
工程技术-材料科学:综合
CiteScore
20.80
自引率
7.30%
发文量
0
审稿时长
23 days
期刊介绍:
The journal Carbon is an international multidisciplinary forum for communicating scientific advances in the field of carbon materials. It reports new findings related to the formation, structure, properties, behaviors, and technological applications of carbons. Carbons are a broad class of ordered or disordered solid phases composed primarily of elemental carbon, including but not limited to carbon black, carbon fibers and filaments, carbon nanotubes, diamond and diamond-like carbon, fullerenes, glassy carbon, graphite, graphene, graphene-oxide, porous carbons, pyrolytic carbon, and other sp2 and non-sp2 hybridized carbon systems. Carbon is the companion title to the open access journal Carbon Trends. Relevant application areas for carbon materials include biology and medicine, catalysis, electronic, optoelectronic, spintronic, high-frequency, and photonic devices, energy storage and conversion systems, environmental applications and water treatment, smart materials and systems, and structural and thermal applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


