心脏装置包膜预防心脏起搏器患者感染的有效性:强化布雷迪现实世界项目
IF 2.9
Q2 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
摘要
与心脏植入式电子装置相关的感染是最具挑战性和最昂贵的并发症之一。虽然可吸收的抗生素洗脱包膜已显示出降低感染风险的功效,但其在起搏器(PM)和心脏再同步化治疗PM (CRT-P)装置中的应用数据有限。目的本研究旨在评估TYRX包膜在PM或CRT-P植入或翻修患者中预防全身和口袋感染的有效性。方法回顾性、多中心分析了896例在2020年8月至2024年4月期间接受PM或CRT-P植入或翻修的患者。将患者分为两组:包膜组(n = 350),接受TYRX包膜,对照组(n = 546)。结果在平均2.2±2.1年的随访中,1.2%的患者发生全身或口袋感染,包膜组感染发生率(0.3%)明显低于对照组(1.8%)(P = 0.05)。包膜与感染风险降低89%相关(发病率比0.11,95%可信区间0.05-0.23,P < 0.001)。Kaplan-Meier分析显示,4年时,包膜组的累计感染发生率为0.4%,对照组为2.5%(风险比为7.36,P = 0.03)。结论在pm和CRT-Ps患者中,使用TYRX包膜可显著降低器械相关感染的发生率。这种益处在长期随访中持续存在,支持其在常规临床实践中的有效性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
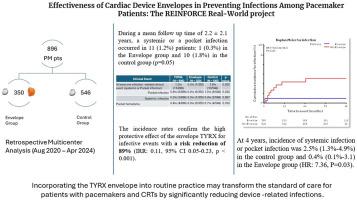
Effectiveness of cardiac device envelopes in preventing infections among pacemaker patients: The REINFORCE Brady real-world project
Background
Infections related to cardiac implantable electrical devices are among the most challenging and costly complications. Although the absorbable antibiotic-eluting envelope has shown efficacy in reducing infection risk, data on its use in pacemaker (PM) and cardiac resynchronization therapy PM (CRT-P) devices are limited.
Objectives
This study aimed to assess the effectiveness of the TYRX envelope in preventing systemic and pocket infections in patients undergoing PM or CRT-P implantation or revision.
Methods
This retrospective, multicenter analysis included 896 patients undergoing PM or CRT-P implantation or revision between August 2020 and April 2024. Patients were categorized into 2 groups: the envelope group (n = 350), receiving the TYRX envelope, and the control group (n = 546).
Results
Over a mean follow-up of 2.2 ± 2.1 years, systemic or pocket infections occurred in 1.2% of patients, with significantly fewer infections in the envelope group (0.3%) than the control group (1.8%) (P = .05). The envelope was associated with an 89% reduction in infection risk (incidence rate ratio 0.11, 95% confidence interval 0.05–0.23, P < .001). Kaplan-Meier analysis showed that, at 4 years, the cumulative incidence of infection was 0.4% in the envelope group vs 2.5% in the control group (hazard ratio 7.36, P = .03).
Conclusions
In patients with PMs and CRT-Ps, the use of the TYRX envelope is associated with significantly lower occurrence of device-related infections. This benefit was sustained over long-term follow-up, supporting its effectiveness in routine clinical practice.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Heart Rhythm O2
Cardiology and Cardiovascular Medicine
CiteScore
3.30
自引率
0.00%
发文量
0
审稿时长
52 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


