锐钛矿和金红石型TiO2晶体界面改善CO氧化反应Pt/TiO2催化剂的耐硫和耐水性能
IF 3.7
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
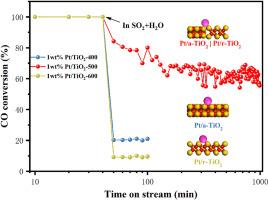
催化氧化是消除CO污染物的有效策略。Pt/TiO2催化剂是目前使用最活跃的催化剂之一,但面临着硫和水失活的问题。在本研究中,TiO2采用溶胶-凝胶法制备,Pt/TiO2采用浸渍法制备。通过改变TiO2载体的煅烧温度,合成了锐钛矿和金红石相比例不同的Pt/TiO2催化剂。在500°C的煅烧温度下,催化剂显示出锐钛矿和金红石的比例大致相等,导致CO氧化的特殊催化活性,以及对烟道气中的硫和水的抗性提高。因此,Pt/TiO2-500催化剂在160℃下的CO转化率达到93%。即使在8% (vol) H2O和0.016% (vol) SO2 (GHSV = 300000 ml·h−1·g−1)的条件下,在220℃下保持46 h, CO转化率仍保持在95%以上。结果表明:锐钛矿相TiO2对CO的吸附能力弱,对SO2的吸附能力强;金红石相TiO2对CO的吸附能力强,对SO2的吸附能力弱;锐钛矿相的存在减轻了催化剂的CO自中毒现象,而两相界面的存在减少了SO2在催化剂表面的吸附和氧化,显著抑制了TiOSO4的沉积。因此,Pt/TiO2-500催化剂表现出最高的CO催化活性,并具有较好的耐硫性和耐水性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The sulfur and water resistance improvement of Pt/TiO2 catalyst for CO oxidation reaction by anatase and rutile TiO2 crystal interfaces
Catalytic oxidation is an effective strategy for eliminating CO pollutant. Pt/TiO2 catalyst are one of the most active catalysts as used, but facing the issue of sulfur and water deactivation. In this study, TiO2 was synthesized using a sol-gel method, while Pt/TiO2 was prepared by impregnation method. By varying the calcination temperature of the TiO2 support, Pt/TiO2 catalysts with different proportions of anatase and rutile phases were synthesized. At the calcination temperature of 500 °C, the catalysts exhibited approximately equal proportions of anatase and rutile, resulting in exceptional catalytic activity for CO oxidation, as well as improved resistance to sulfur and water in the flue gas. Consequently, the Pt/TiO2-500 catalyst achieved a CO conversion of 93% at 160 °C. Even under conditions of 8% (vol) H2O and 0.016% (vol) SO2 (GHSV = 300000 ml·h−1·g−1), the CO conversion remained above 95% at 220 °C for 46 h. The catalysts were characterized and analyzed using various techniques. The results indicated that anatase-phase TiO2 exhibited weak CO adsorption capacity but strong SO2 adsorption capacity, whereas rutile-phase TiO2 demonstrated strong CO adsorption capacity and weak SO2 adsorption capacity. The presence of the anatase phase mitigated the CO self-poisoning phenomenon of the catalyst, while the biphase interface reduced the adsorption and oxidation of SO2 on the catalyst’s surface, significantly inhibiting the deposition of TiOSO4. Consequently, the Pt/TiO2-500 catalyst displayed the highest CO catalytic activity along with superior resistance to sulfur and water.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chinese Journal of Chemical Engineering
工程技术-工程:化工
CiteScore
6.60
自引率
5.30%
发文量
4309
审稿时长
31 days
期刊介绍:
The Chinese Journal of Chemical Engineering (Monthly, started in 1982) is the official journal of the Chemical Industry and Engineering Society of China and published by the Chemical Industry Press Co. Ltd. The aim of the journal is to develop the international exchange of scientific and technical information in the field of chemical engineering. It publishes original research papers that cover the major advancements and achievements in chemical engineering in China as well as some articles from overseas contributors.
The topics of journal include chemical engineering, chemical technology, biochemical engineering, energy and environmental engineering and other relevant fields. Papers are published on the basis of their relevance to theoretical research, practical application or potential uses in the industry as Research Papers, Communications, Reviews and Perspectives. Prominent domestic and overseas chemical experts and scholars have been invited to form an International Advisory Board and the Editorial Committee. It enjoys recognition among Chinese academia and industry as a reliable source of information of what is going on in chemical engineering research, both domestic and abroad.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


