丁腈和酰胺整合的吡唑类分子杂合体:合成、生物学评价和分子对接研究
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
以含芳基和烷基的芳酰肼和酮为原料,采用多级合成技术成功地合成了一组新的腈-羧胺系吡唑衍生物(6a-6g)。在所研究的杂合物中,化合物6d在吡唑核心上具有对氟苯基,在DPPH自由基清除评估中显示出最大的抗氧化活性(~ 83%),与标准抗坏血酸相当。有趣的是,杂种6d对两种类型的胰腺癌SW1990和AsPC1的细胞毒性也有所提高(IC50值分别为18 μM和~ 25 μM)。6d对SW1990和AsPC1癌细胞的活性优于标准药物吉西他滨。分子对接实验进一步证实了它的潜力,显示出与蛋白质BCL-2的强结合亲和力(−8.3 kcal/mol)。目前的研究结果表明,杂交6d是一个很有前途的候选者,它的结构可以作为开发更有效的生物活性化合物的有用模型。本文章由计算机程序翻译,如有差异,请以英文原文为准。
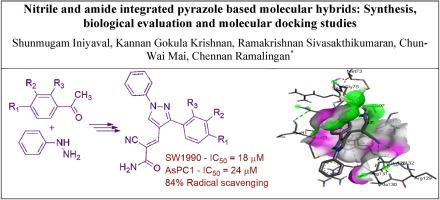
Nitrile and amide integrated pyrazole based molecular hybrids: Synthesis, biological evaluation, and molecular docking studies
A group of new nitrile- and carboxamide-tethered pyrazole derivatives (6a-6g) was successfully synthesized from arylhydrazines and ketones having aryl and alkyl groups using a multi-stage synthesis technique. Of the hybrids studied, the compound 6d, which has a para-fluorophenyl group on the pyrazole core, displayed the maximum antioxidant activity (∼83 %) in the DPPH radical-scavenging evaluation, which is comparable with that of the standard, ascorbic acid. Interestingly, it appears that the hybrid 6d also demonstrated improved cytotoxicity against both cell types of pancreatic cancer, SW1990 and AsPC1 (IC50 values of 18 μM and ∼25 μM, respectively). The activity of 6d is superior to that of the activity of the standard drug, gemcitabine, against both SW1990 and AsPC1 cancer cells. Molecular docking experiments further confirmed its potential, demonstrating a strong binding affinity to the protein, BCL-2 (−8.3 kcal/mol). The current study's findings suggest that the hybrid 6d is a promising candidate and that its structure could serve as a helpful model for developing more potent bioactive compounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


