CDW吸附剂对重金属的吸附:水净化的可持续途径
IF 7.7
Q2 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
有效去除水中重金属对人类健康和环境保护至关重要,吸附剂提供了一种可持续的、具有成本效益的处理解决方案。本研究首次研究了低密度混凝土(LDC)去除水中特定重金属(Pb2+、Co2+和Mn2+)的效果。采用x射线荧光(XRF)、傅里叶变换红外分光光度(FTIR)、布鲁诺尔-埃米特-泰勒(BET)分析、x射线衍射(XRD)分析和场发射扫描电镜(FE-SEM)对吸附剂进行了表征。单因素-一次法(OFAT)优化了影响金属去除的关键参数。采用8种不同的等温线模型确定了吸附参数,阐明了吸附机理。然后分析了它的动力学行为和去污选择性。结果表明,该材料对Pb2+、Mn2+和Co2+的吸附量分别为43.1、23.5和15.2 mg g⁻¹。Pb 2 +、Mn 2 +和Co 2 +的二级动力学速率常数分别为1.99、0.076和0.49 g mg⁻¹min⁻¹。作为一项新的贡献,用泰利斯定理来模拟重金属吸附的再生过程。在本研究中,使用合成废水,而再生条件模拟工业废水,以增强现实世界的相关性。最后,采用可持续材料管理(environmental materials Management, EPA)模型对建筑垃圾吸附剂的应用进行了评价。等温模拟表明,Pb2+、Co2+和Mn2+在最不发达国家废物上的吸附是物理吸附,并且是在非均质表面进行的多层吸附。基于thales的模型表明,经过9次吸附和酸的正式再生循环后,最不发达国家吸附剂的铅浓度始终保持在世界卫生组织(世卫组织)标准以下。本文章由计算机程序翻译,如有差异,请以英文原文为准。
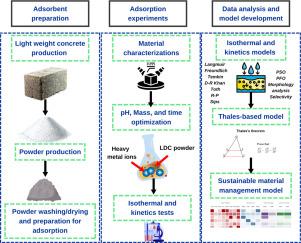
Heavy metals adsorption using CDW adsorbents: A sustainable path for water purification
Effective removal of heavy metals from water is crucial for human health and environmental protection, and adsorbents provide a sustainable, cost-effective treatment solution. This study is the first to investigate Low-Density Concrete (LDC) for removing selected heavy metals (Pb2+, Co2+, and Mn2+) from water. The adsorbent was characterised using X-ray fluorescence (XRF), Fourier Transform Infrared spectrophotometry (FTIR), Brunauer-Emmett-Teller (BET) analysis, X-ray diffraction (XRD) analysis, and Field Emission Scanning Electron Microscopy (FE-SEM). The One-Factor-at-A-Time (OFAT) method optimised the essential parameters affecting the metal removal. Eight different isotherm models were used to determine the adsorption parameters and elucidate the associated sorption mechanism. Then, its kinetic behavior and selectivity for decontamination were analyzed. The outcomes demonstrated that the adsorption capacities for Pb2+, Mn2+, and Co2+ were 43.1, 23.5, and 15.2 mg g⁻¹, respectively. Moreover, the second-order kinetic rate constants for Pb²⁺, Mn²⁺, and Co²⁺ were determined to be 1.99, 0.076, and 0.49 g mg⁻¹ min⁻¹, respectively. As a novel contribution, the regeneration process of heavy metal adsorption is modelled by Thales-based theorem. In this study, synthetic wastewater was used, while regeneration conditions simulated industrial effluents to enhance real-world relevance. Finally, the applied construction and demolition waste (CDW) adsorbent is evaluated by Sustainable Material Management, EPA models. The isothermal modelling illustrated that the adsorption of Pb2+, Co2+, and Mn2+ onto LDC wastes is done physically and on the heterogeneous surface as multilayer adsorption. The Thales-based model demonstrated that after nine cycles of adsorption and formal regeneration with acid, the LDC adsorbent consistently maintained lead concentrations below the World Health Organization (WHO) standard.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of hazardous materials advances
Environmental Engineering
CiteScore
4.80
自引率
0.00%
发文量
0
审稿时长
50 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


