绿度评估的反相高效液相色谱法测定布地奈德纳米颗粒的定量:验证、稳定性和结肠给药的控释
IF 6.2
引用次数: 0
摘要
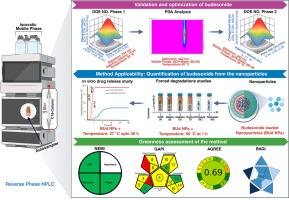
稳健和可持续的分析工具对于设计、制造和评估先进的给药系统至关重要。本研究报告了第一个有效的反相高效液相色谱(RP-HPLC)方法,用于定量布地奈德包封在聚乳酸-羟基乙酸(PLGA)基的、用于炎症性肠病(IBD)结肠递送的逐层(LbL)包被纳米颗粒内。采用响应面法和中心复合设计,在等压模式下确定最佳条件为乙腈:水(80:20,v/v),流速为0.34 mL min-1,检测波长为242 nm,在5 min内完全分离。该方法无缓冲,快速,溶剂高效,导致良好的绿色概况,进一步由GAPI和NEMI评估证实。符合ICH Q2(R2)验证,具有良好的线性(R²> 0.999)、精密度(% RSD < 2%)、准确度、特异性和灵敏度(LOD: 0.04µg mL-1; LOQ: 1.2µg mL-1)。在酸性、碱性、氧化、热、光解和光静条件下的强制降解研究表明,LbL涂层显著提高了药物稳定性,特别是对热和光静胁迫的稳定性,而在碱性和氧化环境下的降解率为75%。在模拟胃肠道条件下的体外释放分析显示,持续的ph响应释放(超过48小时20.6%),与结肠靶向一致。这种经过验证的绿色稳定性指示方法将控释评估与综合性能评估相结合,为纳米颗粒给药系统的质量控制提供了一个通用的平台,并支持其从配方开发到临床转化的进展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Greenness-assessed RP-HPLC method for budesonide quantification in layer-by-layer polymeric nanoparticles: Validation, stability, and controlled release in colonic delivery
Robust and sustainable analytical tools are essential for the design, manufacture, and evaluation of advanced drug delivery systems. This study reports the first validated reversed-phase high-performance liquid chromatography (RP-HPLC) method for quantifying budesonide encapsulated within poly(lactic-co-glycolic acid) (PLGA)-based, layer-by-layer (LbL) coated nanoparticles intended for colonic delivery in inflammatory bowel disease (IBD). Using response surface methodology with a central composite design, optimal conditions were identified as acetonitrile:water (80:20, v/v) under isocratic mode, achieving complete separation within 5 min at a flow rate of 0.34 mL min-1 and detection at 242 nm. The method is buffer-free, rapid, and solvent-efficient, resulting in a favorable greenness profile, further confirmed by GAPI and NEMI assessments. Validation in line with ICH Q2(R2) demonstrated excellent linearity (R² > 0.999), precision (% RSD < 2 %), accuracy, specificity, and sensitivity (LOD: 0.04 µg mL-1; LOQ: 1.2 µg mL-1). Forced degradation studies under acidic, alkaline, oxidative, thermal, photolytic, and photostatic conditions showed that the LbL coating markedly enhanced drug stability, particularly against thermal and photostatic stress, while >75 % degradation occurred in alkaline and oxidative environments. In vitro release profiling under simulated gastrointestinal conditions demonstrated sustained, pH-responsive release (20.6 % over 48 h), consistent with colonic targeting. This validated, green, and stability-indicating method integrates controlled release assessment with comprehensive performance evaluation, providing a versatile platform for quality control of nanoparticulate drug delivery systems and supporting their progression from formulation development to clinical translation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


