Mn(III)-卟啉/透明质酸纳米颗粒通过重塑肿瘤还原微环境增强声动力/化学动力协同治疗
IF 6.3
2区 化学
Q1 POLYMER SCIENCE
引用次数: 0
摘要
超声动力疗法(SDT)和化学动力疗法(CDT)经常受到肿瘤微环境中氧化还原水平升高的限制。巧妙设计的纳米颗粒通过重塑肿瘤微循环来增强声动力/化学动力协同治疗的疗效,是有效的策略。在此,我们通过环糊精修饰的Mn(III)-卟啉和透明质酸(HA)衍生物的自组装构建了能够重塑肿瘤微环境的载药纳米颗粒(HBMD NPs)。包裹在纳米颗粒内的Mn(III)-卟啉消耗内源性谷胱甘肽(GSH),从而有效抑制CDT和SDT产生的活性氧(ROS)的清除。这就显著提高了CDT和SDT联合治疗乳腺癌的疗效。透明质酸作为一种内源性的自靶向载体,显著延长了纳米颗粒的体循环,并扩大了它们在过表达cd44的肿瘤细胞中的积累。纳米颗粒到达酸性肿瘤微环境后,迅速分解并释放金属卟啉。HBMD NPs具有良好的Fenton催化性能和较强的GSH消耗功能,对癌细胞具有显著的细胞毒性和有效的肿瘤抑制作用。本研究通过重塑肿瘤还原性微环境,拓展了SDT/CDT联合治疗癌症的潜在临床应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
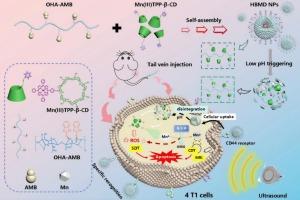
Mn(III)-porphyrin/hyaluronic acid nanoparticles for enhanced sonodynamic/chemodynamic synergistic therapy via remodeling tumor reductive microenvironment
Sonodynamic therapy (SDT) and chemodynamic therapy (CDT) are frequently constrained by the elevated redox levels within the tumor microenvironment. Ingeniously designed nanoparticles that enhance the efficacy of sonodynamic/chemodynamic synergistic therapy by remodeling tumor microcirculation represent effective strategies. Herein, we developed drug-loaded nanoparticles (HBMD NPs) capable of remodeling the tumor microenvironment (TME), which were constructed through self-assembly of cyclodextrin-modified Mn(III)-porphyrin and hyaluronic acid (HA) derivatives. The Mn(III)-porphyrin encapsulated within the nanoparticles depletes endogenous glutathione (GSH), thereby effectively inhibiting the scavenging of reactive oxygen species (ROS) generated by CDT and SDT. This thereby significantly enhances the therapeutic efficacy of combined CDT and SDT against breast cancer. HA functions as an endogenous self-targeting carrier, significantly prolonging the systemic circulation of nanoparticles and amplifying their accumulation in CD44-overexpressing tumor cells. Upon reaching the acidic tumor microenvironment, the nanoparticles rapidly disintegrate and release the metalloporphyrin. The HBMD NPs displayed satisfactory Fenton catalytic properties and a strong GSH depleting function, demonstrating significant cytotoxicity toward cancer cells and efficient tumor inhibition. This work expands the potential clinical applications of combined SDT/CDT for cancer treatment by remodeling the tumor reductive microenvironment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

European Polymer Journal
化学-高分子科学
CiteScore
9.90
自引率
10.00%
发文量
691
审稿时长
23 days
期刊介绍:
European Polymer Journal is dedicated to publishing work on fundamental and applied polymer chemistry and macromolecular materials. The journal covers all aspects of polymer synthesis, including polymerization mechanisms and chemical functional transformations, with a focus on novel polymers and the relationships between molecular structure and polymer properties. In addition, we welcome submissions on bio-based or renewable polymers, stimuli-responsive systems and polymer bio-hybrids. European Polymer Journal also publishes research on the biomedical application of polymers, including drug delivery and regenerative medicine. The main scope is covered but not limited to the following core research areas:
Polymer synthesis and functionalization
• Novel synthetic routes for polymerization, functional modification, controlled/living polymerization and precision polymers.
Stimuli-responsive polymers
• Including shape memory and self-healing polymers.
Supramolecular polymers and self-assembly
• Molecular recognition and higher order polymer structures.
Renewable and sustainable polymers
• Bio-based, biodegradable and anti-microbial polymers and polymeric bio-nanocomposites.
Polymers at interfaces and surfaces
• Chemistry and engineering of surfaces with biological relevance, including patterning, antifouling polymers and polymers for membrane applications.
Biomedical applications and nanomedicine
• Polymers for regenerative medicine, drug delivery molecular release and gene therapy
The scope of European Polymer Journal no longer includes Polymer Physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


