磷酸盐溶液中阳离子的竞争:AZ31镁合金镀层的组成和防护性能
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
研究了磷酸盐溶液中[Na+]/[K+]对AZ31镁合金镀层形成、组成、结构和防护性能的影响。结果表明:涂层机制以局部过饱和和竞争沉淀动力学为基础,受Na+和K+竞争的支配;在[Na+]/[K+] <; 1时,K+取代Na+,生成由Mg(OH)2(水镁石)、MgHPO4·3H2O(新辉石)和KMgPO4·6H2O(鸟粪石-K)组成的单分子膜。在[Na+]/[K+]≥1时,Na+优于K+,形成以新辉石/水镁石为内层,新辉石/水镁石/鸟嘴石为中间层,新辉石/水镁石/鸟嘴石/ KNaMg2(PO4)2·14H2O(黑锰矿)为外层的三层涂层。无论[Na+]/[K+],由于其稳定性差、Na+水化能较高、PO4与Na+尺寸不匹配,NaMgPO4·7H2O(鸟笼石-Na)均未形成,而在[Na+]/[K+] <; 1处,由于Na+水化能较高和过饱和,hazenite的形成被完全抑制。增加[Na+]/[K+]会降低涂层厚度和鸟粪石-K/黑锰矿含量,这是由于晶体成核增强与生长速度的关系。最佳[Na+]/[K+]为1,镀层保护效果最好,腐蚀电流密度降低10倍以上,鸟粪石-K/黑锰矿含量最高。本文章由计算机程序翻译,如有差异,请以英文原文为准。
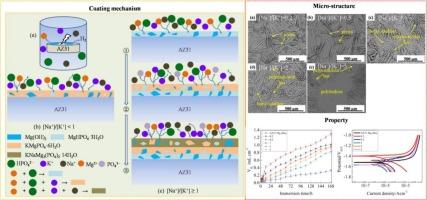
Cation competition in phosphate solution: Tailoring the composition and protectiveness of coatings on AZ31 Mg alloy
The formation, composition, structure and protectiveness of the coatings deposited on AZ31 Mg alloy as a function of [Na+]/[K+] in phosphate solution were systematically investigated. The results showed that the coating mechanism was based on local supersaturation and competitive precipitation kinetics, with the governance of the competition between Na+ and K+. At [Na+]/[K+] < 1, K+ outcompeted Na+, producing a monolayer composed of Mg(OH)2 (brucite), MgHPO4·3H2O (newberyite) and KMgPO4·6H2O (struvite-K). At [Na+]/[K+] ≥ 1, Na+ outcompeted K+, generating a three-layer coating with newberyite/brucite as the inner layer, newberyite/brucite/struvite as the middle layer and newberyite/brucite/struvite/ KNaMg2(PO4)2·14H2O (hazenite) as the outer layer. Regardless of [Na+]/[K+] NaMgPO4·7H2O (struvite-Na) was not formed due to its poor stability, higher hydration energy of Na+ and mismatch between the size of PO4 and Na+, while hazenite formation was completely inhibited at [Na+]/[K+] < 1 due to a higher hydration energy and undersaturation of Na+. Increasing [Na+]/[K+] reduced coating thickness and struvite-K/hazenite content due to enhanced crystal nucleation vs. growth rate. The optimum [Na+]/[K+] was 1, yielding the most protective coating and reducing the corrosion current density of AZ31 Mg alloy by more than 10 times, linked to the highest struvite-K/hazenite content.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


