刚性激活的肝星状细胞通过TGM2/ itgb1介导的基质重塑和线粒体转移促进HCC迁移
IF 7.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
背景:肝硬度高与肝细胞癌(HCC)预后不良相关。先前的研究主要集中在肿瘤细胞而不是肿瘤微环境。本研究探讨了肿瘤微环境,特别是肝星状细胞(hsc)的机械信号传导如何驱动HCC进展。方法采用蛋白质组学、细胞收缩实验和蛋白相互作用等方法研究转谷氨酰胺酶2 (TGM2)和整合素β1 (ITGB1)在机械应力下造血干细胞中的作用。该研究还分析了178例HCC和肝硬化患者的基因表达数据,以评估TGM2和ITGB1对总生存期(OS)的影响。用共聚焦显微镜观察线粒体转移和细胞迁移,并在大鼠肝癌模型中研究TGM2/ITGB1对细胞外基质(ECM)重塑和HCC复发的影响。结果我们发现基质刚度下HSC的激活依赖于ITGB1机械信号,ITGB1激活需要高细胞表面TGM2表达。这个过程激活了下游的CAV1,从而稳定了ITGB1的表达。此外,TGM2/ITGB1高共表达(R = 0.77, p <2.2 × 10-16)与OS呈负相关。有趣的是,我们发现在高硬度条件下通过隧道纳米管在癌症相关成纤维细胞(CAFs)和Huh7细胞之间的杂交共培养中大量线粒体转移(p = 0.0095),这似乎与TGM2/ITGB1有关。在底物硬度增加的情况下,具有cafa来源线粒体的Huh7细胞表现出增强的迁移(p <0.0001)。因此,高肝硬度激活了caf,导致ECM重塑和HCC术后复发。TGM2/ITGB1对于基质刚度驱动的HCC术后复发至关重要。结论本研究揭示了造血干细胞在基质僵硬下促进HCC进展的新机制,这可能有助于HCC临床治疗方案的设计。影响和意义肝星状细胞(hsc)分化为癌症相关成纤维细胞(CAFs),构成肝肿瘤微环境中的主要基质细胞群,并与肝细胞癌(HCC)患者的不良预后相关。CAFs促进HCC进展的确切机制仍未完全阐明。在这项研究中,我们强调了转谷氨酰胺酶2介导的整合素β1在基质硬度增加诱导的造血干细胞活化中的作用。此外,我们探讨了增强的基质硬度如何促进线粒体转移,从而促进HCC细胞的迁移。这些见解可能为HCC临床管理的靶向治疗策略的发展提供信息。本文章由计算机程序翻译,如有差异,请以英文原文为准。
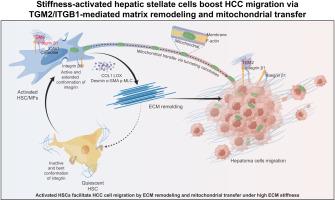
Stiffness-activated hepatic stellate cells boost HCC migration via TGM2/ITGB1-mediated matrix remodeling and mitochondrial transfer
Background & Aims
High liver stiffness correlates with poor outcomes in hepatocellular carcinoma (HCC). Prior studies focused on neoplastic cells rather than the tumor microenvironment. This study investigated how the tumor microenvironment, particularly mechanosignaling in hepatic stellate cells (HSCs), drives HCC progression.
Methods
The study examined the roles of transglutaminase 2 (TGM2) and integrin β1 (ITGB1) in HSCs under mechanical stress through proteomics, cell contraction assays, and protein interactions. It also analyzed gene expression data from 178 patients with HCC and cirrhosis to assess the impact of TGM2 and ITGB1 on overall survival (OS). Mitochondrial transfer and cell migration were observed using confocal microscopy, and the effect of TGM2/ITGB1 on extracellular matrix (ECM) remodeling and HCC recurrence was studied in a rat liver cancer model.
Results
We showed that HSC activation under matrix stiffness relied on ITGB1 mechanosignaling, with high cell-surface TGM2 expression required for ITGB1 activation. This process activated downstream CAV1, which in turn stabilized ITGB1 expression. Moreover, high co-expression of TGM2/ITGB1 (R = 0.77, p <2.2 × 10-16) was negatively correlated with OS. Interestingly, we found massive mitochondrial transfer in hybrid co-cultures between cancer-associated fibroblasts (CAFs) and Huh7 cells by tunneling nanotubes under high stiffness (p = 0.0095), which appeared to be associated with TGM2/ITGB1. Huh7 cells with CAF-derived mitochondria exhibited enhanced migration under increased substrate stiffness (p <0.0001). Accordingly, high liver stiffness activated CAFs, leading to ECM remodeling and postoperative recurrence of HCC. TGM2/ITGB1 was essential for matrix stiffness-driven HCC recurrence following surgery.
Conclusions
This study revealed a novel mechanism by which HSCs facilitate HCC progression under matrix stiffness, which may aid in the design of therapies for the clinical treatment of HCC.
Impact and Implications
Hepatic stellate cells (HSCs) undergo differentiation into cancer-associated fibroblasts (CAFs), which constitute the primary stromal cell population within the liver tumor microenvironment and are associated with poor prognosis in patients with hepatocellular cancer (HCC). The precise mechanisms through which CAFs facilitate HCC progression remain incompletely elucidated. In this study, we emphasize the role of transglutaminase 2-medated integrin β1 in the activation of HSCs induced by increased matrix stiffness. Furthermore, we explore how enhanced matrix stiffness promotes mitochondrial transfer, thereby facilitating the migration of HCC cells. These insights may inform the development of targeted therapeutic strategies for the clinical management of HCC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


