比较两种剂量瑞非尼二线治疗晚期肝细胞癌的随机II期研究
IF 7.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
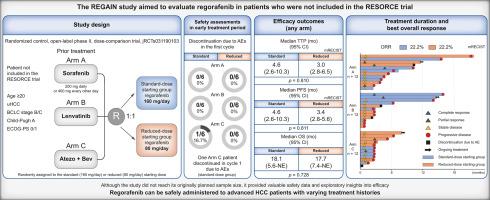
背景和目的:resce试验证实瑞非尼是耐受索拉非尼的晚期肝细胞癌(HCC)患者的二线治疗药物。然而,其在不同治疗史患者中的安全性和有效性尚不清楚。重获研究旨在评估regorafenib在未纳入resce试验的患者中的应用。方法:这项单中心、随机、开放标签、剂量比较试验纳入了先前接受索拉非尼200 mg/天或400 mg每隔一天(A组)、lenvatinib (B组)或atezolizumab加贝伐单抗(C组)治疗的HCC患者。患者被随机分配接受标准(160 mg/天)或减少(80 mg/天)起始剂量的瑞非尼。对于早期安全性评估,在每个剂量组的前6名患者中,由于第一个周期的不良事件(ae)而停药被定义为一种评估措施。主要终点为进展时间(TTP),使用mRECIST评估疗效。结果共纳入36例患者(每组12例)。虽然该研究没有达到最初计划的样本量,但它提供了安全性和探索性疗效数据。1例患者(C组,标准剂量组)因不良反应而在第一个周期停药。标准剂量组和减少剂量组之间的疗效结果具有可比性:中位TTP为4.6个月vs. 3.0个月(p = 0.810),无进展生存期为4.6个月vs. 3.4个月(p = 0.811),总生存期为18.1个月vs. 17.7个月(p = 0.728)。两组患者客观有效率均为22.2%。结论不同治疗史的患者均可安全使用瑞非尼。需要进一步的研究来验证降低起始剂量的临床效用,这可能会优化现实环境中晚期HCC的治疗策略。影响和启示测序和扩大全身治疗可以改善晚期肝细胞癌(HCC)的预后,但关于二线和后续治疗的综合证据仍然有限。本研究评估了regorafenib在现代临床中的应用,包括对索拉非尼不耐受的患者和以前用lenvatinib或atezolizumab加贝伐单抗治疗的患者。Regorafenib的安全剂量为160 mg/天和80 mg/天,探索性疗效分析发现两种起始剂量之间没有显著差异。这些发现支持将瑞非尼整合到晚期HCC的个性化治疗策略中,为专业肝癌治疗的医生提供了更大的灵活性来选择适当的治疗序列,同时也为面临越来越有限的全身治疗选择的患者扩大了治疗选择。临床试验注册jrcts031190103。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Randomized phase II study comparing two doses of regorafenib in second-line treatment for advanced hepatocellular carcinoma
Background & Aims
The RESORCE trial confirmed regorafenib as a second-line treatment for advanced hepatocellular carcinoma (HCC) in patients who tolerated sorafenib. However, its safety and efficacy in patients with diverse treatment histories remain unknown. The REGAIN study sought to assess regorafenib in patients not included in the RESORCE trial.
Methods
This single-center, randomized, open-label, dose-comparison trial included patients with HCC who had previously received sorafenib 200 mg/day or 400 mg every other day (Arm A), lenvatinib (Arm B), or atezolizumab plus bevacizumab (Arm C). Patients were randomly assigned to receive regorafenib at the standard (160 mg/day) or reduced (80 mg/day) starting dose. For early safety assessment, discontinuation as the result of adverse events (AEs) during the first cycle among the first six patients in each dose group was defined as a measure of evaluation. The primary endpoint was time to progression (TTP), and efficacy was evaluated using mRECIST.
Results
Thirty-six patients were enrolled (12 per arm). Although the study did not reach its originally planned sample size, it provided safety and exploratory efficacy data. Discontinuation in the first cycle as a result of AEs was observed in one patient (Arm C, standard-dose group). The efficacy outcomes were comparable between the standard- and reduced-dose groups: median TTP was 4.6 vs. 3.0 months (p = 0.810), progression-free survival was 4.6 vs. 3.4 months (p = 0.811), and overall survival was 18.1 vs. 17.7 months (p = 0.728). The objective response rate was 22.2% in both groups.
Conclusions
Regorafenib can be safely administered with varying treatment histories. Further investigation is warranted to validate the clinical utility of reduced starting dose, which may optimize treatment strategies for advanced HCC in real-world settings.
Impact and implications
Sequencing and extending systemic therapies can improve the prognosis of advanced hepatocellular carcinoma (HCC), but comprehensive evidence on second-line and subsequent treatments remains limited. This study evaluated regorafenib in modern clinical scenarios, including patients intolerant to sorafenib and those previously treated with lenvatinib or atezolizumab plus bevacizumab. Regorafenib was safely administered at 160 mg/day and 80 mg/day, and an exploratory efficacy analysis found no significant difference between the two starting doses. These findings support the integration of regorafenib into personalized treatment strategies for advanced HCC, providing physicians specializing in liver cancer treatment with greater flexibility in selecting appropriate therapeutic sequences while also expanding treatment options for patients facing increasingly limited systemic therapy choices.
Clinical Trials Registration
jRCTs031190103.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


