基于结构的新型琥珀酸脱氢酶抑制剂β-酮腈衍生物的分子设计
IF 4
1区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
琥珀酸脱氢酶抑制剂(SDHI)是化学杀菌剂中最重要的一类,可有效防治植物病害。在SDHI杀菌剂研究中,β-酮腈已被确定为一种新的药效支架。本研究基于与SDH的结合模式,合理设计合成了一系列创新的含β-酮腈的联苯片段化合物,旨在加强芳香相互作用,提高杀真菌活性。最佳目标化合物B24和B29对番茄根核菌和菌核菌具有显著的体外杀真菌活性,EC50值分别为0.096和0.072 μg/mL。B24对水稻纹枯病有一定的防治作用,B29对油菜菌核病有较好的防治效果,进一步验证了B24对水稻纹枯病的体内保护性杀真菌活性。此外,这两种化合物都表现出有效的SDH抑制活性,B24的IC₅0值为0.042 μM, B29的IC₅0值为0.030 μM。分子对接研究进一步表明,联苯基团的掺入通过形成t形π相互作用或π-π堆叠相互作用增强了配体与靶芳香族的相互作用,从而提高了结合亲和力和杀真菌活性。与氟吡虫沙相比,化合物B24和B29对非靶水生生物、水蚤和大水蚤的毒性也较低。本研究确定了具有创新潜力的β-酮腈杀菌剂先导化合物,可用于后续优化,为农用SDHI杀菌剂的研究与开发提供有价值的线索。本文章由计算机程序翻译,如有差异,请以英文原文为准。
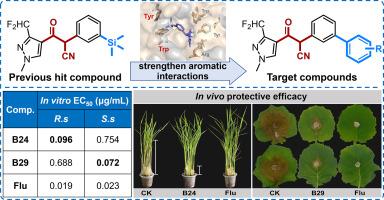
Structure-based molecular design of fungicidal β-ketonitrile derivatives with biphenyl moiety as novel succinate dehydrogenase inhibitors
Succinate dehydrogenase inhibitor (SDHI) remains one of the most important subclasses of chemical fungicides for the effective control of plant diseases. β-Ketonitrile has been identified as a novel pharmacophoric scaffold in SDHI fungicide research. In this study, a series of innovative biphenyl moiety containing β-ketonitrile compounds were rationally designed and synthetized based on the binding mode with SDH, aiming to strengthen the aromatic interactions and improve the fungicidal activities. The optimal target compounds B24 and B29 exhibited significant in vitro fungicidal activities against Rhizoctonia solani and Sclerotiorum sclerotiorum, with EC50 values of 0.096 μg/mL and 0.072 μg/mL, respectively. Furthermore, the promising in vivo protective fungicidal activities were further validated, with B24 showed certain activity against rice sheath blight and B29 effectively controlled oilseed rape sclerotinia disease. In addition, both compounds demonstrated potent SDH inhibitory activities, with IC₅₀ values of 0.042 μM for B24 and 0.030 μM for B29, respectively. Molecular docking studies further demonstrated that the incorporation of biphenyl moieties enhanced ligand-target aromatic interactions through forming T-shaped π interaction or π-π stacking interaction, thereby contributing to the improved binding affinity and fungicidal activity. Compounds B24 and B29 also exhibited lower toxicities to non-target aquatic organisms, Danio rerio and Daphnia magna, compared to fluxapyroxad. The present work identified potential innovative β-ketonitrile fungicidal lead compounds for following optimization, giving valuable clues to the research and development of agricultural SDHI fungicides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.00
自引率
8.50%
发文量
238
审稿时长
4.2 months
期刊介绍:
Pesticide Biochemistry and Physiology publishes original scientific articles pertaining to the mode of action of plant protection agents such as insecticides, fungicides, herbicides, and similar compounds, including nonlethal pest control agents, biosynthesis of pheromones, hormones, and plant resistance agents. Manuscripts may include a biochemical, physiological, or molecular study for an understanding of comparative toxicology or selective toxicity of both target and nontarget organisms. Particular interest will be given to studies on the molecular biology of pest control, toxicology, and pesticide resistance.
Research Areas Emphasized Include the Biochemistry and Physiology of:
• Comparative toxicity
• Mode of action
• Pathophysiology
• Plant growth regulators
• Resistance
• Other effects of pesticides on both parasites and hosts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


