零间隙碳酸氢盐(CO2)电解的双极膜三维微晶格电极组件设计
IF 7.1
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
与传统的气相方法相比,从碳捕获溶液(如(bi)碳酸盐)中电化学还原二氧化碳(eCO2R)为节能碳回收提供了一条有前途的途径。然而,优化膜电极设计的发展仍然是推动该技术走向商业可行性的关键挑战。在这项研究中,我们提出了一种新的3d打印微晶格碳电极与双极膜(bpm)的集成,用于碳酸氢盐电解。我们使用投影微立体光刻技术(PμSL)制作了独特的独立碳电极,其结构灵感来自于大自然的蜂巢结构。在不同的沉积电位(-0.1、-0.2和-0.5 V vs Ag/AgCl)、沉积时间(0 ~ 1200 s)和催化剂负载(0 ~ 0.7 mg/cm²)条件下,通过计时电流法将银纳米颗粒沉积到电极上。优化的BPM-3D电极组件显著提高了CO的生产效率,在12.5 mA/cm²的电流密度下,CO (FECO)的法拉第效率达到38%,比未涂层电极(1.7%)提高了22倍。该结构在-0.2 V下制备900 s, Ag负载为0.5 mg/cm²,由于形成了高活性的Ag纳米结构,因此表现出更高的催化活性。该电极在50 mA/cm²时的综合催化性能指数(ICPI)约为150 mA/Vmg。稳定性测试显示,在14小时的运行中,ag涂层3D电极的退化最小,证实了其耐久性。初步的技术经济分析表明,在规模化条件下,优化法拉第效率(FE)和催化剂负载可以降低CO的生产成本。这项工作为整合3D打印和BPM技术以实现可持续eCO₂R奠定了基础。然而,在100ma /cm²下实现高选择性和稳定性仍然具有挑战性,需要优化电极设计和界面工程以实现商业可行性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
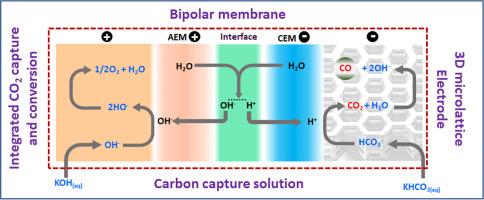
Bipolar membrane 3D microlattice electrode assembly design for zero-gap bicarbonate (CO2) electrolysis
The electrochemical reduction of CO2 (eCO2R) from carbon capture solutions, such as (bi)carbonate, offers a promising pathway for energy-efficient carbon recycling compared to traditional gas-phase approaches. However, the development of optimized membrane-electrode designs remains a critical challenge for advancing this technology toward commercial viability. In this study, we present a novel integration of 3D-printed microlattice carbon electrodes with bipolar membranes (BPMs) for bicarbonate electrolysis. We used projection micro-stereolithography (PμSL) to fabricate unique free-standing carbon electrodes with nature-inspired honeycomb architectures. Silver nanoparticles were deposited onto the electrodes via chronoamperometry under varying conditions of deposition potential (-0.1, -0.2, and -0.5 V vs Ag/AgCl), deposition time (0 to 1200 s), and catalyst loading (0 to 0.7 mg/cm²). The optimal BPM-3D electrode assembly significantly enhanced CO production efficiency, achieving a Faradaic efficiency for CO (FECO) of 38 % - a 22 times increase over uncoated electrodes (1.7 %) - at a current density of 12.5 mA/cm². This configuration, prepared at -0.2 V for 900 s with an Ag loading of 0.5 mg/cm², exhibited improved catalytic activity because of the formation of highly active Ag nanostructures. The electrode demonstrated an Integrated Catalytic Performance Index (ICPI) of approximately 150 mA/Vmg at 50 mA/cm². Stability tests revealed minimal degradation over 14 h of operation, affirming the durability of the Ag-coated 3D electrodes. A preliminary techno-economic analysis projects that CO production costs could be reduced by optimizing Faradaic efficiency (FE) and catalyst loading under scaled conditions. This work lays the foundation for integrating 3D printing and BPM technology for sustainable eCO₂R. However, achieving high selectivity and stability at >100 mA/cm² remains challenging, requiring optimized electrode design and interface engineering for commercial viability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal Advances
Engineering-Industrial and Manufacturing Engineering
CiteScore
8.30
自引率
0.00%
发文量
213
审稿时长
26 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


