含有α -硫辛酸和芦丁的热敏原位凝胶用于治疗角膜新生血管:配方、表征和体内评价
IF 4.9
3区 医学
Q1 PHARMACOLOGY & PHARMACY
Journal of Drug Delivery Science and Technology
Pub Date : 2025-09-16
DOI:10.1016/j.jddst.2025.107538
引用次数: 0
摘要
角膜新生血管(CnV)是一种重要的眼科疾病,经常导致不适和视力损害。由于传统药物治疗CnV的局限性和副作用,人们正在探索创新的治疗策略。在这项研究中,评估了α -硫辛酸和芦丁负载的热敏原位凝胶治疗CnV的潜力。以壳聚糖、波洛沙姆407和波洛沙姆188为聚合物,采用冷法制备眼原位凝胶。采用实验设计(DoE)方法研究配方变量对关键质量属性的影响,包括pH、凝胶温度和粘度。优化后的载药凝胶pH值为5.05±0.1,在25℃条件下具有341±26 cP的伪塑性流动特性,在34±0.4℃条件下具有凝胶性。药物释放符合威布尔动力学和一级动力学。通过L929和ARPE-19细胞系的体外细胞毒性研究证实了其眼部安全性。大鼠体内研究和角膜组织化学分析表明,优化后的配方(配方K)与CnV标准治疗地塞米松一样有效,并进一步促进上皮细胞再生。眼部组织的组织学评估显示,K配方组的炎症和新生血管减少,支持其治疗潜力。总的来说,我们的研究结果表明,开发的原位凝胶系统具有持续的药物释放和增强的治疗效果,可用于治疗角膜新生血管。本文章由计算机程序翻译,如有差异,请以英文原文为准。
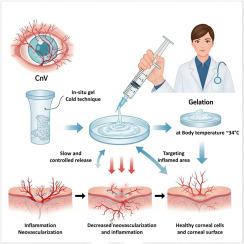
Thermosensitive in situ gel containing alpha-lipoic acid and rutin for the treatment of corneal neovascularization: formulation, characterization, and in vivo evaluation
Corneal neovascularization (CnV) is a significant ophthalmological condition often leading to discomfort and vision impairment. Due to the limitations and side effects associated with conventional drug therapies for CnV, innovative treatment strategies are being explored. In this study, the potential of alpha-lipoic acid and rutin-loaded thermosensitive in situ gels for the treatment of CnV was evaluated. The ocular in situ gel was formulated using the cold method and included chitosan, poloxamer 407, and poloxamer 188 as polymers. A design of experiments (DoE) approach was employed to investigate the effects of formulation variables on critical quality attributes, including pH, gelation temperature, and viscosity. The optimized drug-loaded gel exhibited a pH of 5.05 ± 0.1, pseudo-plastic flow behavior with a viscosity of 341 ± 26 cP at 25 °C, and gelation at 34 ± 0.4 °C. Drug release followed Weibull and first-order kinetics. Ocular safety was confirmed through in vitro cytotoxicity studies using L929 and ARPE-19 cell lines. In vivo studies in rats and histochemical analyses of the cornea demonstrated that the optimized formulation (Formulation K) was as effective as dexamethasone, a standard treatment for CnV, and further promoted epithelial cell regeneration. Histological evaluations of ocular tissues revealed reduced inflammation and neovascularization in the Formulation K group, supporting its therapeutic potential. Overall, our findings suggest that the developed in situ gel system offers sustained drug release and enhanced therapeutic efficacy for the treatment of corneal neovascularization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.00
自引率
8.00%
发文量
879
审稿时长
94 days
期刊介绍:
The Journal of Drug Delivery Science and Technology is an international journal devoted to drug delivery and pharmaceutical technology. The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on controlled release, bioavailability and drug absorption, nanomedicines, gene delivery, tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


