新型空心氮化硼纳米结构
IF 5.1
3区 材料科学
Q2 MATERIALS SCIENCE, COATINGS & FILMS
引用次数: 0
摘要
这项工作介绍了一种新的中空氮化硼(BN)纳米结构家族,它被设计成与Sundholm及其同事报道的碳基高迪烯结构类似的等电子结构。B36N36、B108N108、B216N216和B324N324笼通过用BN对取代碳对中的CC键构建。GFN2-xTB计算通过B36N36的DFT验证,确认所有结构都是稳定的最小值,具有较大的HOMO-LUMO间隙。化学键分析揭示了氮原子周围的电子定位和非芳香性。紧密结合的分子动力学模拟显示了高达1000 K的优异热稳定性,所有系统都保持其空心拓扑结构;B216N216笼具有优越的回弹性。在高温下,较大的体系经历了向稳定的六方BN基序的尺寸依赖转变,伴随着显著的能量稳定。这些结果建立了一类具有纳米技术应用前景的新型BN纳米结构。本文章由计算机程序翻译,如有差异,请以英文原文为准。
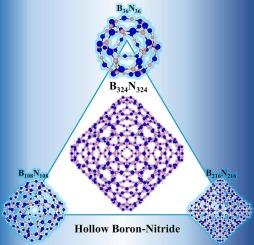
Novel hollow boron-nitride nanostructures
This work introduces a new family of hollow boron nitride (BN) nanostructures, designed as isoelectronic analogs to the carbon-based Gaudiene architectures reported by Sundholm and co-workers. The B36N36, B108N108, B216N216 and B324N324 cages were constructed by replacing C![]() C bonds in their carbon counterparts with B
C bonds in their carbon counterparts with B![]() N pairs. GFN2-xTB calculations, validated by DFT for B36N36, confirm all structures as stable minima with large HOMO–LUMO gaps. Chemical bonding analysis reveals electron localization around nitrogen atoms and a non-aromatic character. Tight-binding molecular dynamics simulations demonstrate exceptional thermal stability up to 1000 K, with all systems retaining their hollow topology; the B216N216 cage exhibits superior resilience. At high temperatures, larger systems undergo a size-dependent transformation toward stable hexagonal BN motifs, accompanied by significant energy stabilization. These results establish a novel class of BN nanostructures with promising properties for nanotechnology applications.
N pairs. GFN2-xTB calculations, validated by DFT for B36N36, confirm all structures as stable minima with large HOMO–LUMO gaps. Chemical bonding analysis reveals electron localization around nitrogen atoms and a non-aromatic character. Tight-binding molecular dynamics simulations demonstrate exceptional thermal stability up to 1000 K, with all systems retaining their hollow topology; the B216N216 cage exhibits superior resilience. At high temperatures, larger systems undergo a size-dependent transformation toward stable hexagonal BN motifs, accompanied by significant energy stabilization. These results establish a novel class of BN nanostructures with promising properties for nanotechnology applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Diamond and Related Materials
工程技术-材料科学:综合
CiteScore
6.00
自引率
14.60%
发文量
702
审稿时长
2.1 months
期刊介绍:
DRM is a leading international journal that publishes new fundamental and applied research on all forms of diamond, the integration of diamond with other advanced materials and development of technologies exploiting diamond. The synthesis, characterization and processing of single crystal diamond, polycrystalline films, nanodiamond powders and heterostructures with other advanced materials are encouraged topics for technical and review articles. In addition to diamond, the journal publishes manuscripts on the synthesis, characterization and application of other related materials including diamond-like carbons, carbon nanotubes, graphene, and boron and carbon nitrides. Articles are sought on the chemical functionalization of diamond and related materials as well as their use in electrochemistry, energy storage and conversion, chemical and biological sensing, imaging, thermal management, photonic and quantum applications, electron emission and electronic devices.
The International Conference on Diamond and Carbon Materials has evolved into the largest and most well attended forum in the field of diamond, providing a forum to showcase the latest results in the science and technology of diamond and other carbon materials such as carbon nanotubes, graphene, and diamond-like carbon. Run annually in association with Diamond and Related Materials the conference provides junior and established researchers the opportunity to exchange the latest results ranging from fundamental physical and chemical concepts to applied research focusing on the next generation carbon-based devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


