Fe-Cu/HZSM-5催化类fenton体系降解罗丹明B
IF 6.3
3区 工程技术
Q1 ENGINEERING, CHEMICAL
Journal of the Taiwan Institute of Chemical Engineers
Pub Date : 2025-09-18
DOI:10.1016/j.jtice.2025.106412
引用次数: 0
摘要
屈丹明B (RhB)对水生生态系统构成严重威胁。Fenton氧化法是一种很有前途的降解RhB的方法,但传统Fenton反应存在pH范围窄、可重复使用性差的问题。为了克服这些问题,本研究开发了Fe-Cu/HZSM-5催化剂的类fenton体系,以有效降解RhB。方法采用浸渍法制备FexCu5-x/HZSM-5系列催化剂(x为Fe与Cu的摩尔比)。将这些催化剂与H2O2结合,构建类fenton体系降解RhB。采用XRD、NH3-TPD、XPS、FT-IR、N2低温吸附-脱附等技术对催化剂进行了表征。在测试的催化剂中,Fe3Cu2/HZSM-5表现出最佳的性能,在45°C、5.3 mM/L H2O2、3.0 g/L催化剂用量和较宽的pH范围(2-5)下,可以几乎完全降解RhB(~ 100%)。机理研究证实,羟基自由基(·OH)是类芬顿体系中RhB降解的主要氧化剂。在·OH的攻击下,RhB发生n -去乙基化和共轭结构破坏等一系列反应,最终矿化成CO2和H2O。总之,Fe-Cu/HZSM-5催化剂的开发解决了传统Fenton反应的局限性,为该领域的未来研究提供了有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
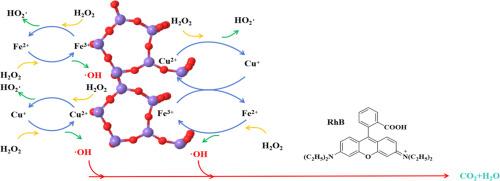
Degradation of rhodamine B by fenton-like system with Fe-Cu/HZSM-5 catalyst
Background
Rhodamine B (RhB) poses a significant threat to aquatic ecosystems. While Fenton oxidation process is promising approach for RhB degradation, the conventional Fenton reaction has the narrow pH range and poor reusability. To overcome these, this study developed Fenton-like system with Fe-Cu/HZSM-5 catalyst for effective RhB degradation.
Methods
A series of FexCu5-x/HZSM-5 catalysts (x represents the molar ratio of Fe to Cu) were prepared by impregnation. These catalysts were integrated with H2O2 to construct Fenton-like system for the degradation of RhB. The catalysts were characterized by XRD, NH3-TPD, XPS, FT-IR, and N2 adsorption-desorption at low temperature techniques.
Significant findings
Among the tested catalysts, Fe3Cu2/HZSM-5 exhibited optimal performance, achieving near-complete RhB degradation (∼100%) under the condition of 45 °C, 5.3 mM/L H2O2, 3.0 g/L catalyst dosage, and broad pH range (2–5). Mechanistic studies confirmed that hydroxyl radicals (·OH) were the primary oxidants responsible for RhB degradation in Fenton-like system. Under ·OH attacking, RhB underwent a series of reactions such as N-deethylation and disruption of the conjugated structure and was eventually mineralized into CO2 and H2O. Overall, a novel Fe-Cu/HZSM-5 catalyst was developed to address the limitations of the traditional Fenton reaction, providing valuable insights for future research in this field.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.10
自引率
14.00%
发文量
362
审稿时长
35 days
期刊介绍:
Journal of the Taiwan Institute of Chemical Engineers (formerly known as Journal of the Chinese Institute of Chemical Engineers) publishes original works, from fundamental principles to practical applications, in the broad field of chemical engineering with special focus on three aspects: Chemical and Biomolecular Science and Technology, Energy and Environmental Science and Technology, and Materials Science and Technology. Authors should choose for their manuscript an appropriate aspect section and a few related classifications when submitting to the journal online.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


