在藜麦中,ABA协调连接抗氧化防御、糖代谢和昼夜节律时钟的分子网络,以实现综合应激耐受性。
IF 5.7
2区 生物学
Q1 PLANT SCIENCES
引用次数: 0
摘要
藜麦(Chenopodium Quinoa wild .)是一种新兴的非生物抗逆性模式物种,由于其特殊的环境适应性,它对低温-干旱联合胁迫表现出独特的分子适应性。然而,ABA增强藜麦组合抗逆性的机制尚不清楚。本研究利用综合生理和转录组学分析对藜麦中脱落酸(ABA)介导的调控网络进行了表征。我们发现,复合胁迫通过显著上调NCED - ABA生物合成基因(log2(Fold Change) = 2.37至4.98,p < 0.05)而抑制CYP707A - ABA分解代谢基因(log2(Fold Change) = - 1.81至- 2.99,p < 0.05)触发ABA积累。低温干旱胁迫下外源ABA激活了PYR/PYL-PP2C-SnRK2级联,增强了抗氧化防御(SOD、POD、CAT),增加了可溶性糖与淀粉的比例,减轻了氧化损伤。通过WGCNA鉴定的aba响应抗氧化模块(MEwhite, 110个基因)协调氧化还原稳态和碳水化合物代谢。该模块依靠以hsf为中心的转录网络来增强抗逆性。该模块整合ERF和WRKY转录因子协调应激适应,同时增强抗氧化酶活性清除ROS,维持组合应激下的代谢平衡。至关重要的是,昼夜节律基因(CRY, GI, PRR5)协调aba依赖和aba独立的途径,增强应激适应,揭示应激信号和生物钟之间的串扰。氟啶酮介导的ABA抑制加重了氧化还原酶活性和可溶性糖含量的抑制,证实了ABA的关键作用。这些发现为aba驱动的组合胁迫适应提供了机制见解,为培育气候适应型作物提供了目标。本文章由计算机程序翻译,如有差异,请以英文原文为准。
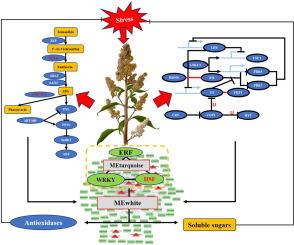
ABA orchestrates molecular networks linking antioxidant defense, sugar metabolism and circadian clock for combined stress tolerance in Quinoa (Chenopodium quinoa Willd.)
Quinoa (Chenopodium quinoa Willd.), an emerging model species for abiotic stress tolerance due to its exceptional environmental resilience, exhibits unique molecular adaptations to combined low temperature-drought stress. However, the mechanism by which ABA enhances combined stress tolerance in quinoa remains elusive. This study characterized the abscisic acid (ABA)-mediated regulatory network in quinoa using integrated physiological and transcriptomics analyses. We demonstrated that combined stress triggers ABA accumulation via significant up-regulating NCED - ABA biosynthesis genes (log2(Fold Change) = 2.37 to 4.98, p < 0.05) while suppressing CYP707A - ABA catabolism genes (log2(Fold Change) = −1.81 to −2.99, p < 0.05). Exogenous ABA application under low temperature-drought stress activated the PYR/PYL-PP2C-SnRK2 cascade, thereby enhancing antioxidant defenses (SOD, POD, CAT) and increasing the ratio of soluble sugars to starch, which mitigated oxidative damage. The ABA-responsive antioxidant module (MEwhite, 110 genes), identified via WGCNA, coordinates redox homeostasis and carbohydrate metabolism. This module relies on HSF-centered transcriptional networks to enhance stress tolerance. This module integrates ERF and WRKY transcription factors to coordinate stress adaptation, concurrently enhancing antioxidant enzyme activity for ROS scavenging while maintaining metabolic equilibrium under combinatorial stress. Crucially, circadian rhythm genes (CRY, GI, PRR5) coordinates both ABA-dependent and ABA-independent pathways enhance stress adaptation, revealing crosstalk between stress signaling and biological clocks. Fluridone-mediated ABA suppression exacerbated the inhibition of oxidoreductase activity and soluble sugar content, confirming ABA's pivotal role. These findings provide mechanistic insights into ABA-driven combinatorial stress adaptation, offering targets for breeding climate-resilient crops.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.10
自引率
3.10%
发文量
410
审稿时长
33 days
期刊介绍:
Plant Physiology and Biochemistry publishes original theoretical, experimental and technical contributions in the various fields of plant physiology (biochemistry, physiology, structure, genetics, plant-microbe interactions, etc.) at diverse levels of integration (molecular, subcellular, cellular, organ, whole plant, environmental). Opinions expressed in the journal are the sole responsibility of the authors and publication does not imply the editors'' agreement.
Manuscripts describing molecular-genetic and/or gene expression data that are not integrated with biochemical analysis and/or actual measurements of plant physiological processes are not suitable for PPB. Also "Omics" studies (transcriptomics, proteomics, metabolomics, etc.) reporting descriptive analysis without an element of functional validation assays, will not be considered. Similarly, applied agronomic or phytochemical studies that generate no new, fundamental insights in plant physiological and/or biochemical processes are not suitable for publication in PPB.
Plant Physiology and Biochemistry publishes several types of articles: Reviews, Papers and Short Papers. Articles for Reviews are either invited by the editor or proposed by the authors for the editor''s prior agreement. Reviews should not exceed 40 typewritten pages and Short Papers no more than approximately 8 typewritten pages. The fundamental character of Plant Physiology and Biochemistry remains that of a journal for original results.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


