用最小的RISC复合物包封细胞外囊泡作为新型基因沉默工具
引用次数: 0
摘要
近年来,包括小干扰rna (sirna)和反义寡核苷酸(ASOs)在内的基因沉默方式在基础研究和临床应用中都得到了蓬勃发展,而传递平台是成功的关键因素之一。细胞外囊泡(Extracellular vesic泡,ev)作为细胞间通讯的天然载体,已被设计成多种方式作为基因沉默的传递平台,但其效率和可重复性有限。在这项研究中,我们开发了一种新的策略,将电动汽车作为基因沉默工具。由修饰Argonaute 2 (AGO2)蛋白和专门设计的引导链rna组成的最小rna诱导沉默复合体(RISC)被封装到ev中,并在概念验证研究中引起了显著的EGFP沉默。这种模块化的EVs平台,我们将其命名为minRISC-EVs,有效地沉默了M1巨噬细胞中iNOS的表达以及M2巨噬细胞中STAT6/A20的表达,使巨噬细胞向所需方向极化。在体内进一步验证了巨噬细胞调节能力,在脂多糖(LPS)诱导的急性肺损伤模型中,minRISC-EVs抗iNOS减轻小鼠肺部炎症,在肿瘤异种移植模型中,minRISC-EVs抗STAT6/A20抑制B16F10肿瘤进展。综上所述,minrisc - ev可以作为一种新的基因沉默工具,在未来的临床转化中具有很大的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
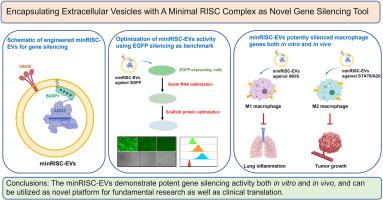
Encapsulating extracellular vesicles with a minimal RISC complex as novel gene silencing tool
Gene silencing modalities including small interfering RNAs (siRNAs) and antisense oligonucleotides (ASOs) have prospered in both fundamental research and clinical translations in recent years, with delivery platform being one of the key elements for success. Extracellular vesicles (EVs) as natural carriers for cell-cell communication have been engineered in a variety of ways as delivery platform for gene silencing, yet facing limited efficiency and reproducibility. In this study, we developed a new strategy for engineering EVs as gene silencing tool. A minimal RNA-induced silencing complex (RISC), composing of modified Argonaute 2 (AGO2) protein and specially designed guide strand RNAs, were encapsulated into EVs and elicited prominent EGFP silencing in proof-of-concept study. This modular EVs platform, which we named as minRISC-EVs, efficiently silenced iNOS expression in M1 macrophages as well as STAT6/A20 expression in M2 macrophages, enabling macrophages polarization towards desired directions. The macrophage modulating ability was further validated in vivo, as minRISC-EVs against iNOS alleviated mice lung inflammation in lipopolysaccharide (LPS)-induced acute lung injury model, and minRISC-EVs against STAT6/A20 inhibited B16F10 tumor progression in the tumor xenograft model. In summary, minRISC-EVs can be utilized as novel gene silencing tool, and hold great promise for clinical translation in the future.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Extracellular vesicle
Biochemistry, Genetics and Molecular Biology (General)
自引率
0.00%
发文量
0
审稿时长
43 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


