新型人用IVIG制剂Yimmugo®的安全性验证
IF 4
Q2 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
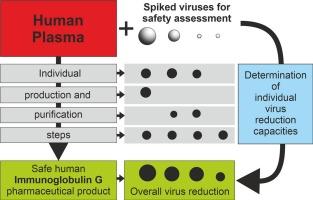
静脉输注人IgG (IVIG)对原发性或继发性免疫缺陷综合征患者至关重要,但在临床上也发现对其他具有自身免疫原性或炎症背景的疾病有益。近几十年来,其临床应用和需求不断上升。除了IgG,其他人类抗体类别也已进入临床应用,因此,一种新的制造工艺已被设想,可以同时分离两种免疫球蛋白制剂,Yimmugo™(IVIG)和trimodulin (IgM浓缩物)。由于这些抗体的唯一合适来源是人类供体血浆,抗体是通过一系列专门的纯化步骤从血浆中分离出来的,因此存在将传染性人类病原体带入最终制备的风险。我们在这里描述了为提供Yimmugo所采取的措施的验证,Yimmugo是一种IVIG产品,具有强大的生物安全性,不含微生物、病毒或朊病毒来源的病原体。为此,我们在Yimmugo的生产过程中间体中加入了多种病原体,并测试了生产步骤从制剂中清除这些病原体的能力。我们表明,四种不同的纯化步骤的Yimmugo程序有效地授予清除病毒和朊病毒病原体,从而提供了一个安全的产品,即使在假设的情况下,在原始材料中存在传染因子。因此,新的制备程序产生可证明安全的产品,并同时允许从相同的血浆池生产额外的药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Validation of adventitious agent safety in Yimmugo®, a novel IVIG preparation for human use
Intravenous infusion of human IgG (IVIG) is of vital importance for patients suffering from primary or secondary immunodeficiency syndromes, but also has been found to be clinically beneficial in other diseases with autoimmunogenic or inflammatory background. Its clinical application and therefore its demand have been continuously rising during the last decades. Besides IgG, other human antibody classes have also entered clinical applications, therefore a novel manufacturing procedure has been conceived which allows simultaneous isolation of two immunoglobulin preparations, Yimmugo™ (an IVIG) and trimodulin (an IgM concentrate). Since the only suitable source for these antibodies is human donor plasma, from which it is isolated using a series of dedicated purification steps, there is a risk of carrying over infectious human pathogens into the final preparations. We describe here validation of the measures taken to provide Yimmugo, an IVIG product, with robust margins of biological safety, free of pathogens of microbial, viral or prion origin. To this end, we spiked manufacturing process intermediates of Yimmugo with diverse pathogens and tested the capacity of the manufacturing steps to clear these from the preparation. We show that four different purification steps of the Yimmugo procedure efficiently confer clearance of viral and prion pathogens, thereby providing a safe product even in the hypothetical case that an infectious agent in the original material were present. Consequently, the novel preparation procedure yields a provably safe product and simultaneously allows production of an additional medicine from the same plasma pool.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Current Research in Biotechnology
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
6.70
自引率
3.60%
发文量
50
审稿时长
38 days
期刊介绍:
Current Research in Biotechnology (CRBIOT) is a new primary research, gold open access journal from Elsevier. CRBIOT publishes original papers, reviews, and short communications (including viewpoints and perspectives) resulting from research in biotechnology and biotech-associated disciplines.
Current Research in Biotechnology is a peer-reviewed gold open access (OA) journal and upon acceptance all articles are permanently and freely available. It is a companion to the highly regarded review journal Current Opinion in Biotechnology (2018 CiteScore 8.450) and is part of the Current Opinion and Research (CO+RE) suite of journals. All CO+RE journals leverage the Current Opinion legacy-of editorial excellence, high-impact, and global reach-to ensure they are a widely read resource that is integral to scientists' workflow.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


