替扎尼定与美洛昔康超分子自组装协同增强抗偏头痛疗效
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
偏头痛是一种影响很大一部分人口的衰弱性疾病,在治疗管理中提出了一个复杂的挑战。基于超分子自组装的药物-药物共结晶是一种很有前途的策略,因为它不仅改善了活性药物成分的物理化学性质,而且还释放了它们的协同临床潜力。本研究通过晶体工程设计替扎尼定和美洛昔康的超分子自组装体,探讨它们在抗偏头痛治疗中的联合临床价值。得到的多组分晶体替扎尼丁-美洛昔康通过晶体学、光谱学和热分析进行了全面表征。它形成一个稳定的电荷辅助氢键超分子网络,比替扎尼定的溶解度提高~60 %,比美洛昔康的溶解度提高 ~ 10倍。在大鼠偏头痛模型中,它达到100% %完全缓解,持续镇痛是物理混合物的1.3倍(P <; 0.05)。此外,溶液化学结果表明,替扎尼丁-美洛昔康盐的增强治疗性能可能归因于其缓释速度,更高的平衡溶解度,独特的分子构象以及两种化合物之间更强的相互作用。替扎尼丁-美洛昔康盐在水溶液中能形成均匀稳定的分子团簇,进一步提高了其疗效和稳定性。该研究表明,药物多组分晶体是开发具有更高疗效的新型制剂的重要策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
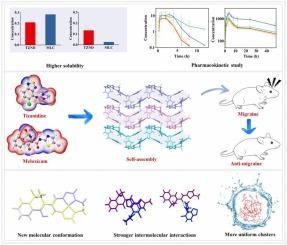
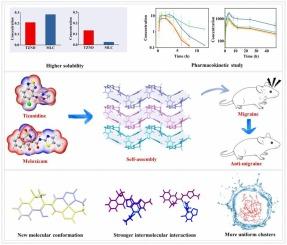
Synergistic enhancement of anti-migraine efficacy through supramolecular self-assembly of tizanidine with meloxicam
Migraine, a debilitating medical condition that affects a significant portion of the population, presents a complex challenge in therapeutic management. Drug–drug cocrystallization based on supramolecular self-assembly offers a promising strategy, as it not only improves the physicochemical properties of active pharmaceutical ingredients, but also unlocks their synergistic clinical potential. In this study, supramolecular self-assemblies of tizanidine and meloxicam were designed through crystal engineering to explore their combined clinical value in anti-migraine therapy. The resulting multicomponent crystal, tizanidine-meloxicam, was thoroughly characterized by crystallography, spectroscopy, and thermal analysis. It forms a stable charge-assisted hydrogen bond supramolecular network, exhibiting ∼60 % improved solubility over tizanidine and ∼ 10-fold enhancement over meloxicam. In a rat migraine model, it achieved 100 % complete remission, with sustained analgesia 1.3 times greater than the physical mixture (P < 0.05). Furthermore, solution chemistry results indicate that the enhanced therapeutic performance of tizanidine-meloxicam salt can be attributed to its sustained release rate, higher equilibrium solubility, distinct molecular conformation, and stronger interactions between the two compounds. Additionally, tizanidine-meloxicam salt could form uniform and stable molecular clusters in aqueous solution, which could further enhance its efficacy and stability. This study demonstrates that pharmaceutical multicomponent crystals is a significant strategy for the development of novel formulations with improved efficacy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


