基于喹唑啉的双靶点抑制剂破坏流感病毒RNP复合物:有效抗流感药物的合理设计、合成和机制验证
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
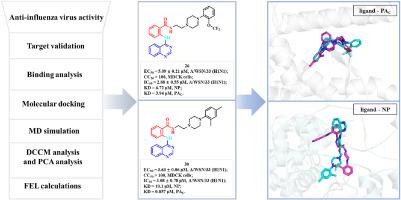
在这项研究中,我们报道了34种针对流感病毒核糖核蛋白(RNP)复合物的新型喹唑啉类似物的合理设计和合成。这些化合物被分为四个结构类,它们的抗流感活性被评估。先导化合物26和30对A/WSN/33(H1N1)具有较强的抗病毒作用,EC50值分别为5.09±0.21 μM和3.63±0.06 μM,选择性指数高(SI >; 19.7和>; 27.5)。表面等离子体共振(SPR)实验证实了病毒RNP复合物与核蛋白(NP, KD = 6.72 μM, 26 μM, 10.1 μM, 30 μM)和PAc端结构域(PAc, KD = 3.94 μM, 26 μM, 0.857 μM, 30 μM)的双重结合。分子对接和动力学模拟揭示了关键的相互作用:破坏NP Glu339-Arg416盐桥(对寡聚化至关重要)并结合到PA-PB1界面(残基Lys643, Glu623, Trp706),破坏聚合酶组装。RNP抑制实验进一步证实了病毒转录/复制的抑制作用(在12.5 μM下抑制56.8-68.5%)。尽管具有良好的效力,但溶解度优化对于改善药物样性质仍然是必要的。通过将静态对接姿态与md衍生的动态相关性(DCCM)、主成分分析(PCA)和fel量化的能量盆地相结合,该研究揭示了短暂但在机械上至关重要的配体-蛋白质相互作用节点。本研究确立了喹唑啉类双靶向抑制剂作为抗流感药物的前景,为进一步开发抗耐药菌株奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Quinazoline-based dual-target inhibitors disrupt influenza virus RNP complex: Rational design, synthesis and mechanistic validation of potent anti-influenza agents
In this study, we report the rational design and synthesis of 34 novel quinazoline analogs targeting the influenza virus ribonucleoprotein (RNP) complex. These compounds, categorized into four structural classes, were evaluated for anti-influenza activity. Lead compounds 26 and 30 demonstrated potent antiviral efficacy against A/WSN/33(H1N1), with EC50 values of 5.09 ± 0.21 μM and 3.63 ± 0.06 μM, respectively, and high selectivity indices (SI > 19.7 and > 27.5). Surface plasmon resonance (SPR) experiments confirmed dual binding to nucleoprotein (NP; KD = 6.72 μM for 26, 10.1 μM for 30) and the PA C-terminal domain (PAc; KD = 3.94 μM for 26, 0.857 μM for 30), key components of the viral RNP complex. Molecular docking and dynamics simulations revealed critical interactions: disruption of the NP Glu339-Arg416 salt bridge (essential for oligomerization) and binding to the PA-PB1 interface (residues Lys643, Glu623, Trp706), destabilizing polymerase assembly. RNP inhibition assays further validated suppression of viral transcription/replication (56.8–68.5 % inhibition at 12.5 μM). Despite favorable potency, solubility optimization remains necessary for improved drug-like properties. By integrating static docking poses with MD-derived dynamic correlations (DCCM), principal component analysis (PCA), and FEL-quantified energy basins, this study revealed transient yet mechanistically vital ligand-protein interaction nodes. This study establishes quinazoline-based dual-targeting inhibitors as promising anti-influenza agents, providing a foundation for further development against resistant strains.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


