温度和溶质调节BSA溶液的液-液分离。
IF 3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
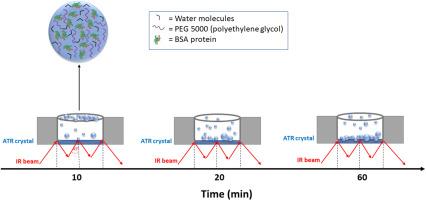
生物分子凝聚物是在拥挤的细胞环境中形成的,在结构复杂性低或无序区域大的大分子中更受青睐。在温度、pH、压力和组分浓度等特定实验条件下,也可以在体外观察到富含大分子区域的蛋白质溶液的液-液相分离(LLPS)。在本研究中,我们研究了聚乙二醇(PEG-5000)和温度诱导牛血清白蛋白(BSA) LLPS的形成。通过温度依赖浊度、光学显微镜和红外光谱实验对BSA溶液的LLPS和液滴形成进行了评估和表征。结果表明,PEG浓度越低,BSA溶液的LLPS转变温度越低。当PEG浓度为10% (w/v)时,10℃时BSA液滴的平均直径约为9 μm, 20℃时降至3 μm左右,温度升高时液滴溶解,形成均匀相。在实时形成BSA液滴之后,还进行了ATR-FTIR动力学实验,可以估计液滴中的蛋白质浓度,其值比初始溶液(100 μM)高50倍。与均相相比,凝析物中的蛋白质二级结构没有变化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Temperature and cosolute regulate the liquid-liquid phase separation in BSA solutions
Biomolecular condensates form in vivo in the crowded cellular environment and are favoured in macromolecules containing low structural complexity or large portions of disordered regions. The liquid-liquid phase separation (LLPS) of protein solutions, in which macromolecule-rich regions are separated from the aqueous solution, can also be observed in vitro under specific experimental conditions of temperature, pH, pressure and components concentration. In this study, we investigate the formation of LLPS of bovine serum albumin (BSA) induced by polyethylene glycol (PEG-5000) and temperature. The LLPS of BSA solutions and droplets formation were assessed and characterized by temperature dependent turbidity, optical microscopy and infrared spectroscopy experiments. The results show that the lower the PEG concentration, the lower the LLPS transition temperature of BSA solution. At PEG concentration of 10 % (w/v) the average diameter of BSA droplets is about 9 μm at 10 °C, decreases to about 3 μm at 20 °C and at higher temperature the droplets dissolve and a homogeneous phase is observed. The real time formation of the BSA droplets is also followed by ATR-FTIR kinetic experiments that allow an estimate of protein concentration in the droplets giving a value 50 times higher than the initial solution (100 μM). No variation of the protein secondary structure within the condensates compared to the homogeneous phase is evidenced.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


