黄药活化醇光氧化催化杜鹃花的C-H脱氧烷基化反应
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在三环己基膦的存在下,利用黄药盐作为烷基化前驱体,建立了一种可见光驱动的氮化脲与醇的脱氧烷基化方法。该方法具有条件温和、台阶效率高、底物范围广的特点,可以使用伯、仲、叔醇作为烷基化剂。此外,机理研究表明,光氧化还原介导的自由基途径涉及黄药中间体,从而提高了反应的选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。

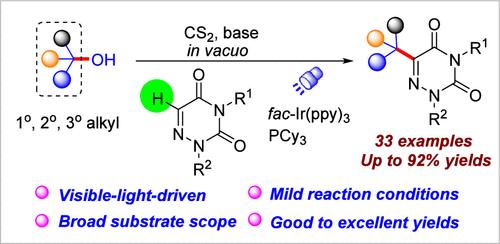
Photoredox-Catalyzed Deoxygenative C–H Alkylation of Azauracils via Xanthate-Activated Alcohols
A visible-light-driven method has been developed for the deoxygenative alkylation of azauracils with alcohols, utilizing xanthate salts as alkylation precursors in the presence of tricyclohexylphosphine. This approach features mild conditions, high step efficiency, and a broad substrate scope, enabling the use of primary, secondary and tertiary alcohols as alkylating agents. Additionally, mechanistic investigations suggest a photoredox-mediated radical pathway that involves xanthate intermediates, thereby enhancing the reaction selectivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


