结直肠癌细胞中新型GPX4抑制剂牛蒡子素衍生物的设计、合成和生物学评价
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
谷胱甘肽过氧化物酶4 (GPX4)是铁下垂的关键调节因子,已成为癌症治疗的一个有吸引力的治疗靶点。利用这一靶点的治疗潜力,我们通过构效关系(SAR)研究,合理设计合成了25个新的牛蒡素衍生物。值得注意的是,化合物W25对HCT-116结直肠癌细胞的抗肿瘤作用最强,超过了母体化合物arctigenin的活性。流式细胞术分析显示,W25使HCT-116细胞的脂质活性氧(ROS)水平提高了4.5倍。值得注意的是,铁下垂抑制剂(ferrostatin-1和甲磺酸去铁胺)可以逆转W25诱导的脂质ROS的增加,这表明W25可以选择性地触发HCT-116细胞的铁下垂。同时,W25抑制HCT-116细胞中谷胱甘肽(GSH)水平,升高丙二醛(MDA)水平。更重要的是,Western blotting结果显示,W25对GPX4蛋白的表达呈剂量依赖性,而对其他凋亡相关蛋白(如FSP1、FTH1、SLC7A11)无明显影响。酶活性数据也显示W25能抑制GPX4的活性。此外,MG132(一种蛋白酶体抑制剂)逆转了W25对GPX4蛋白表达的抑制。相应的,免疫沉淀实验也显示W25促进了GPX4的泛素化水平,表明W25诱导了GPX4的泛素化依赖性蛋白酶体降解。这些结果表明W25是一种新型的通过抑制GPX4来抑制结直肠癌的铁下垂诱导剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
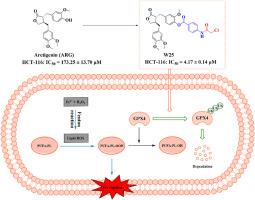
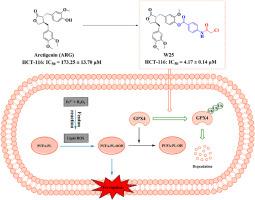
Design, synthesis, and biological evaluation of arctigenin derivatives as novel GPX4 inhibitors in colorectal cancer cells
Glutathione peroxidase 4 (GPX4), a critical regulator of ferroptosis, has emerged as an attractive therapeutic target for cancer therapy. Leveraging the therapeutic potential of this target, we rationally designed and synthesized 25 novel arctigenin derivatives through structure-activity relationship (SAR) studies. Remarkably, compound W25 displayed the most potent antitumor efficacy against HCT-116 colorectal cancer cells, surpassing the activity of the parent compound arctigenin. Flow cytometry analysis revealed that W25 increased lipid reactive oxygen species (ROS) levels by 4.5 times in HCT-116 cells. Notably, ferroptosis inhibitors (ferrostatin-1 and deferoxamine mesylate) could reverse the increase of lipid ROS induced by W25, suggesting that W25 could selectively trigger ferroptosis in HCT-116 cells. Meanwhile, W25 suppressed the level of glutathione (GSH) and increased the level of malondialdehyde (MDA) in HCT-116 cells. More importantly, the Western blotting results displayed that W25 inhibited the protein expression of GPX4 in a dose-dependent manner, while it had no significant effect on other ferroptosis-related proteins (such as FSP1, FTH1, and SLC7A11). Enzyme activity data also showed that W25 could suppress the activity of GPX4. Furthermore, MG132 (a proteasome inhibitor) reversed the inhibition of GPX4 protein expression by W25. Correspondingly, the immunoprecipitation assay also showed that W25 promoted the ubiquitination level of GPX4, revealing that W25 induced ubiquitination-dependent proteasomal degradation of GPX4. These results suggest that W25 is a novel ferroptosis inducer against colorectal cancer through the inhibition of GPX4.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


