利用工程自适应纳米颗粒调节巨噬细胞治疗角膜碱烧伤
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
角膜碱烧伤是一种严重的眼科急症,其特征是进行性炎症、上皮细胞再生受损和病理性新生血管形成,带来了重大的临床挑战。传统的局部治疗受到角膜前快速清除和角膜渗透有限的阻碍。为了克服这些限制,我们设计了自适应纳米粒子(PDNPs),由聚唾液酸(PSA)-地塞米松(Dex)通过酸敏感腙键自组装形成。局部给药后,psa介导的siglece识别使PDNPs选择性锚定在浸润的角膜基质巨噬细胞上,然后这些巨噬细胞进行趋化定向迁移到更深的炎症组织,增强眼表保留和药物渗透。在损伤角膜的酸性微环境中,PDNPs的Dex释放受到控制,从而实现对巨噬细胞的精确时空调节。在碱损伤小鼠模型中,PDNPs显著延长眼表滞留时间,改善间质药物分布。此外,PDNPs治疗可显著加速角膜再上皮化,恢复基质透明度,减少角膜水肿,抑制新生血管形成。机制研究表明,PDNPs通过有效抑制角膜炎症的关键轴MAPK/NF-κB信号通路减轻角膜损伤。总的来说,PDNPs表现出良好的治疗效果和耐受性,作为调节炎症性眼表疾病的新平台具有潜在的应用前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
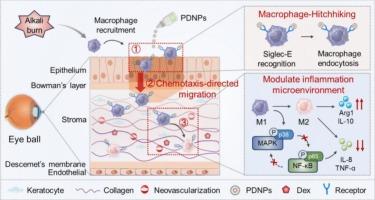
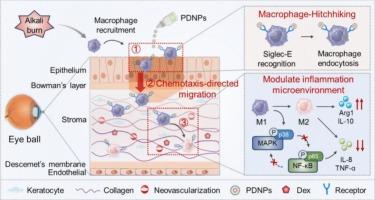
Harnessing engineered adaptive nanoparticles to modulate macrophages for treating corneal alkali burns
Corneal alkali burns represent a severe ophthalmic emergency characterized by progressive inflammation, impaired epithelial regeneration, and pathological neovascularization, posing significant clinical challenges. Conventional topical therapies are hindered by rapid precorneal clearance and limited corneal penetration. To overcome these limitations, we designed adaptive nanoparticles (PDNPs) formed by the self-assembly of polysialic acid (PSA)-dexamethasone (Dex) via acid-sensitive hydrazone linkages. Upon topical administration, PSA-mediated Siglec-E recognition enabled selective anchoring of PDNPs to infiltrating corneal stromal macrophages, which then undergo chemotaxis-directed migration into deeper inflamed tissues, enhancing ocular surface retention and drug penetration. In the acidic microenvironment of the injured cornea, PDNPs undergo controlled Dex release, enabling precise spatiotemporally modulation of macrophages. In mice model of alkali injury, PDNPs significantly prolonged ocular surface retention and improved stromal drug distribution. Moreover, the treatment of PDNPs remarkedly accelerated corneal re-epithelialization, restored stromal transparency, reduce corneal edema and inhibit neovascularization. Mechanistic study indicated that PDNPs alleviated the corneal injury by effectively inhibited of the MAPK/NF-κB signaling pathway, a key axis in corneal inflammation. Overall, PDNPs demonstrated excellent therapeutic efficacy and tolerability for potential application as a novel platform for modulating inflammatory ocular surface diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


